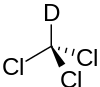
Deuterated chloroform
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Trichloro(2H)methane | |||
| Other names
Chloroform-d Deuterochloroform | |||
| Identifiers | |||
| 1697633 | |||
| 865-49-6 | |||
| ChEBI | CHEBI:85365 | ||
| ChemSpider | 64654 | ||
| EC number | 212-742-4 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 71583 | ||
| |||
| UN number | 1888 | ||
| Properties | |||
| CDCl3 | |||
| Molar mass | 120.384 g mol−1 | ||
| Density | 1.500 g cm−3 | ||
| Melting point | −64 °C (−83 °F; 209 K) | ||
| Boiling point | 61 °C (142 °F; 334 K) | ||
| Hazards | |||
| EU classification | | ||
| R-phrases | R22, R38, R40, R48/20/22 | ||
| S-phrases | S36/37 | ||
| NFPA 704 | |||
| Related compounds | |||
| Related compounds |
Chloroform | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Deuterated chloroform (CDCl3), is an isotopologue of chloroform (CHCl3) in which the hydrogen atom ("H") is replaced with a deuterium (heavy hydrogen) isotope ("D"). Deuterated chloroform is a common solvent used in NMR spectroscopy of organic molecules.[1] In proton NMR spectroscopy, the deuterium does not exhibit a large interfering peak, whereas protium (regular hydrogen) shows a large peak in the spectrum. In carbon-13 NMR, the sole carbon deuterated chloroform shows a triplet at a chemical shift of 77 ppm with the three peaks being about equal size.[1]
References
| ||||||||||