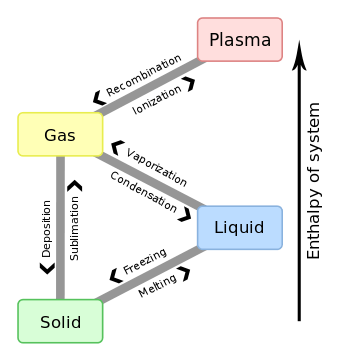
Deposition (phase transition)

Deposition, also known as desublimation, is a thermodynamic process, a phase transition in which gas transforms into solid. The reverse of deposition is sublimation.
One example of deposition is the process by which, in sub-freezing air, water vapor changes directly to ice without first becoming a liquid. This is how snow forms in clouds, as well as frost and hoar frost on the ground. Another example is when frost forms on a leaf. For deposition to occur, thermal energy must be removed from a gas. When the leaf becomes cold enough, water vapor in the air surrounding the leaf loses enough thermal energy to change into a solid. Deposition in water vapor occurs due to the pureness of the water vapor. The vapor has no foreign particles, and is therefore able to lose large amounts of energy before forming around something. When the leaf is introduced, the supercooled water vapor immediately begins to condense, but by this point is already past the freezing point. This causes the water vapor to change directly into a solid.
Another example of physical deposition is the artificial process of physical vapor deposition, used to deposit thin films of various materials onto various surfaces.
Deposition releases energy and is an exothermic phase change.
References
- Jacobson, Mark Z., Fundamentals of Atmospheric Modeling, Cambridge University Press, 2nd ed., 2005, p. 525 ISBN 978-0-521-83970-9
- Moore, John W., et al., Principles of Chemistry: The Molecular Science, Brooks Cole, 2009, p. 387 ISBN 978-0-495-39079-4
- Whitten, Kenneth W., et al., Chemistry, Brooks-Cole, 9th ed., 2009, p. 7 ISBN 978-0-495-39163-0
- Glencoe Science "Focus on Physical Science"
| ||||||||||||||||||||||||||||||||||||