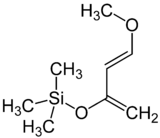
Danishefsky's diene
 | |
 | |
| Names | |
|---|---|
| Other names
Kitahara diene trans-1-Methoxy-3-trimethylsilyloxy-1,3-butadiene (E)-1-Methoxy-3-trimethylsilyloxy-1,3-butadiene | |
| Identifiers | |
| 54125-02-9 | |
| ChemSpider | 4518295 |
| |
| Jmol-3D images | Image |
| PubChem | 5366448 |
| |
| Properties | |
| Molecular formula |
C8H16O2Si |
| Molar mass | 172.30 g·mol−1 |
| Density | 0.89 g cm−3 (20 °C)[2] |
| Boiling point | 68 °C (154 °F; 341 K) at 0.0189 kPa[2] |
| Hazards | |
| R-phrases | - |
| S-phrases | S23, S24/25 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Danishefsky’s diene (Kitahara diene) is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky.[3][4] Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines,[5] aldehydes, alkenes and alkynes.[4] Reactions with imines [6] and nitro-olefins [7] have been reported.
It was first synthesized by the reaction of trimethylsilyl chloride with 4-methoxy-3-buten-2-one and zinc chloride:[8]
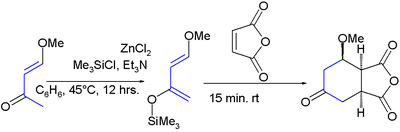
The diene has two features of interest: the substituents promote regiospecific addition to unsymmetrical dienophiles and the resulting adduct is amenable to further functional group manipulations after the addition reaction. High regioselectivity is obtained with unsymmetrical alkenes with a preference for a 1,2-relation of the ether group with the electron-deficient alkene-carbon. All this is exemplified in this Aza Diels-Alder reaction:[9][10]

In the cycloaddition product, the silyl ether is a synthon for a carbonyl group via the enol. The methoxy group is susceptible to an elimination reaction enabling the formation of a new alkene group.
Applications in asymmetric synthesis have been reported.[11][12][13][14][15][16][17][18][19][20][21][22] Derivatives have been reported.[23]
References
- ↑ Sigma-Aldrich product page.
- ↑ 2.0 2.1 Sicherheitsdatenblatt Merck.
- ↑ Samuel J. Danishefsky; Kitahara, T. Useful diene for the Diels-Alder reaction. J. Am. Chem. Soc. 1974, 96, 7807-7808. doi:10.1021/ja00832a031
- ↑ 4.0 4.1 Strategic Applications of Named Reactions in Organic Synthesis, Laszlo Kurti,Barbara Czako, Elsevier 2005
- ↑ Acid-Free Aza Diels−Alder Reaction of Danishefsky's Diene with Imines Yu Yuan,Xin Li, and, and Kuiling Ding Organic Letters 2002 4 (19), 3309-3311 doi:10.1021/ol0265822
- ↑ Alkaline salt-catalyzed aza Diels–Alder reactions of Danishefsky’s diene with imines in water under neutral conditions Catherine Loncaric, Kei Manabea and Shū Kobayashi Chem. Commun., 2003, 574-575 doi:10.1039/B300880K
- ↑ Exo selective Diels–Alder reaction of nitroolefins with Danishefsky's diene Manabu Node, Kiyoharu Nishide, Hitoshi Imazato, Ryuichi Kurosaki, Takehisa Inoue and Takao Ikariya Chem. Commun., 1996, 2559-2560 doi:10.1039/CC9960002559
- ↑ Preparation and Diels-Alder Reaction of a Highly Nuclerophilic Diene. Org. Synth., Coll. Vol. 7, p.312 (1990); Vol. 61, p.147 (1983). Link
- ↑ Asymmetric aza-Diels-Alder reaction of Danishefsky's diene with imines in a chiral reaction medium Pegot B, Nguyen Van Buu O, Gori D, Vo-Thanh G Beilstein Journal of Organic Chemistry, 2006 Link
- ↑ This is an asymmetric reaction with a chiral ionic liquid as chiral solvent. The reported chemical yield is 66% with 60% diastereomeric excess
- ↑ Development of an unusually highly enantioselective hetero-Diels-Alder reaction of benzaldehyde with activated dienes catalyzed by hypercoordinating chiral aluminum complexes Simonsen KB1, Svenstrup N, Roberson M, Jorgensen KA. Chemistry. 2000 Jan;6(1):123-8. PubMed
- ↑ Catalytic enantioselective aza-Diels-alder reactions of imines--an approach to optically active nonproteinogenic alpha-amino acids Yao S, Saaby S, Hazell RG, Jorgensen KA. Chemistry. 2000 Jul 3;6(13):2435-48. PubMed
- ↑ Total syntheses of the Securinega alkaloids (+)-14,15-dihydronorsecurinine, (−)-norsecurinine, and phyllanthine. Han G, LaPorte MG, Folmer JJ, Werner KM, Weinreb SM. J Org Chem. 2000 Oct 6;65(20):6293-306. PubMed
- ↑ Racemic and asymmetric Diels-Alder reactions of 1-(2-oxazolidinon-3-yl)-3-siloxy-1,3-butadienes. Janey JM, Iwama T, Kozmin SA, Rawal VH.J Org Chem. 2000 Dec 29;65(26):9059-68.PubMed
- ↑ Total asymmetric synthesis of the putative structure of the cytotoxic diterpenoid (−)-sclerophytin a and of the authentic natural sclerophytins A and B. Bernardelli P, Moradei OM, Friedrich D, Yang J, Gallou F, Dyck BP, Doskotch RW, Lange T, Paquette LA. J Am Chem Soc. 2001 Sep 19;123(37):9021-32. doi:10.1021/ja011285y
- ↑ A straightforward stereoselective synthesis of D- and L-5-hydroxy-4-hydroxymethyl-2-cyclohexenylguanine. Wang J, Morral J, Hendrix C, Herdewijn P.J Org Chem. 2001 Dec 14;66(25):8478-82. doi:10.1021/jo015924z
- ↑ Chiral hetero Diels-Alder products by enantioselective and diastereoselective zirconium catalysis. Scope, limitation, mechanism, and application to the concise synthesis of (+)-Prelactone C and (+)-9-deoxygoniopypyrone. Yamashita Y, Saito S, Ishitani H, Kobayashi S.J Am Chem Soc. 2003 Apr 2;125(13):3793-8. doi:10.1021/ja028186k
- ↑ Enantioselective catalysis of hetero Diels-Alder reaction and diethylzinc addition using a single catalyst. Du H, Ding K.Org Lett. 2003 Apr 3;5(7):1091-3. doi:10.1021/ol034143c
- ↑ Asymmetric hetero Diels-Alder reactions of Danishefsky's diene and glyoxylate esters catalyzed by chiral bisoxazoline derived catalysts Arun K. Ghosh, Packiarajan Mathivanan,John Cappiello,K. Krishnan Tetrahedron: Asymmetry Volume 7, Issue 8, August 1996, Pages 2165–2168 doi:10.1016/0957-4166(96)00261-3
- ↑ Asymmetric Hetero-Diels–Alder Reaction of Danishefsky’s Dienes with α-Carbonyl Esters Catalyzed by an Indium(III)–PyBox Complex Bei Zhao and Teck-Peng Loh Organic Letters 2013 15 (12), 2914-2917 doi:10.1021/ol400841s
- ↑ A Highly Enantioselective Hetero-Diels−Alder Reaction of Aldehydes with Danishefsky's Diene Catalyzed by Chiral Titanium(IV) 5,5‘,6,6‘,7,7‘,8,8‘-Octahydro-1,1‘-bi-2-naphthol Complexes Bin Wang, Xiaoming Feng, Yaozong Huang, Hui Liu, Xin Cui and Yaozhong Jiang The Journal of Organic Chemistry 2002 67 (7), 2175-2182 doi:10.1021/jo016240u
- ↑ Zheng, J., Lin, L., Fu, K., Zhang, Y., Liu, X. and Feng, X. (2014), Asymmetric Hetero-Diels–Alder Reaction of Danishefsky’s Diene with α-Ketoesters and Isatins Catalyzed by a Chiral N,N′-Dioxide/Magnesium(II) Complex. Chem. Eur. J., 20: 14493–14498. doi:10.1002/chem.201404144
- ↑ Difluorinated Danishefsky's diene: a versatile C(4) building block for the fluorinated six-membered rings. Amii H, Kobayashi T, Terasawa H, Uneyama K. Org Lett. 2001 Oct 4;3(20):3103-5. doi:10.1021/ol0163631