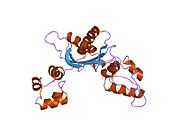
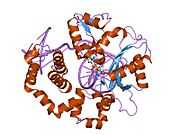
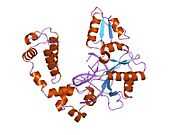
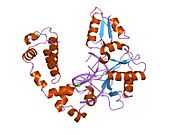
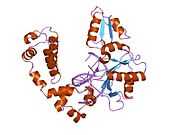
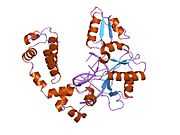
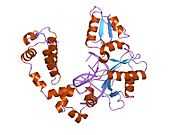
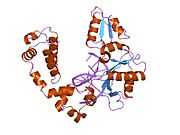
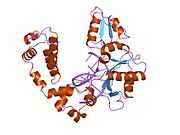
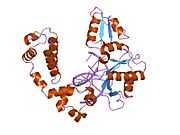
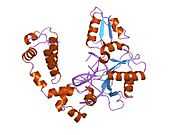
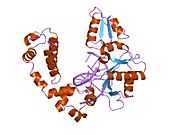
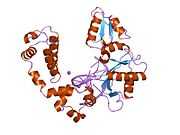
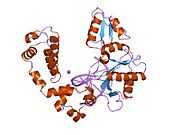
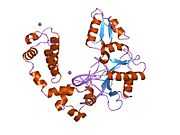
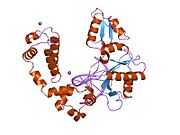
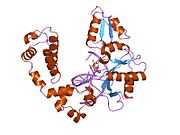
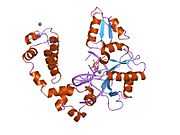
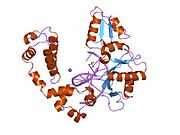
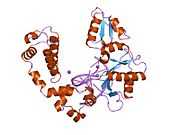
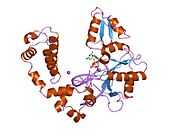
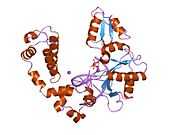
DNA polymerase beta
| Stem loopII regulatory element in POLB | |
|---|---|
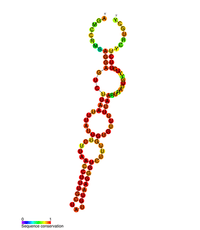 | |
| Predicted secondary structure of the stem loopII (M2) regulatory element in POLB | |
| Identifiers | |
| Symbol | POLB |
| Rfam | RF01455 |
| Entrez | 5423 |
| HUGO | POLB |
| OMIM | 174760 |
| RefSeq | NM_002690 |
| Other data | |
| RNA type | Cis-reg |
| Domain(s) | Mammalia |
| Locus | Chr. 8 p11.2 |
Polymerase (DNA directed), beta, also known as POLB, is an enzyme that, in humans, is encoded by the POLB gene.[1]
Function
In eukaryotic cells, DNA polymerase beta (POLB) performs base excision repair (BER) required for DNA maintenance, replication, recombination, and drug resistance.[1]
Regulation of expression
DNA polymerase beta maintains genome integrity by participating in base excision repair. Overexpression of POLB mRNA has been correlated with a number of cancer types, whereas deficiencies in POLB results in hypersensitivity to alkylating agents, induced apoptosis, and chromosomal breaking [ref7]. Therefore, it is essential that POLB expression is tightly regulated.[2][3][4][5]
POLB gene is upregulated by CREB1 transcription factor's binding to the cAMP response element(CRE) present in the promoter of the POLB gene in response to exposure to alkylating agents.[6][7] POLB gene expression is also regulated at the post transcriptional level as the 3’UTR of the POLB mRNA has been shown to contain three stem-loop structures that influence gene expression.[8] These three-stem loop structures are known as M1, M2, and M3, where M2 and M3 have a key role in gene regulation. M3 contributes to gene expression, as it contains the polyadenylation signal followed by the cleavage and polyadenylation site, thereby contributing to pre-mRNA processing. M2 has been shown to be evolutionary conserved, and, through mutagenesis, it was shown that this stem loop structure acts as a RNA destabilizing element.
In addition to these cis-regulatory elements present within the 3’UTR a trans-acting protein, HAX1 is thought to contribute to the regulation of gene expression. Yeast three-hybrid assays have shown that this protein binds to the stem loops within the 3’UTR of the POLB mRNA, however the exact mechanism in how this protein regulates gene expression is still to be determined.
Interactions
DNA polymerase beta has been shown to interact with PNKP[9] and XRCC1.[10][11][12][13]
See also
- POLA1
- POLA2
References
- ↑ 1.0 1.1 "Entrez Gene: POLB polymerase (DNA directed), beta".
- ↑ Canitrot Y; Cazaux C; Fréchet M et al. (October 1998). "Overexpression of DNA polymerase beta in cell results in a mutator phenotype and a decreased sensitivity to anticancer drugs". Proc. Natl. Acad. Sci. U.S.A. 95 (21): 12586–90. doi:10.1073/pnas.95.21.12586. PMC 22874. PMID 9770529.
- ↑ Bergoglio V; Pillaire MJ; Lacroix-Triki M et al. (June 2002). "Deregulated DNA polymerase beta induces chromosome instability and tumorigenesis". Cancer Res. 62 (12): 3511–4. PMID 12067997.
- ↑ Bergoglio V; Canitrot Y; Hogarth L et al. (September 2001). "Enhanced expression and activity of DNA polymerase beta in human ovarian tumor cells: impact on sensitivity towards antitumor agents". Oncogene 20 (43): 6181–7. doi:10.1038/sj.onc.1204743. PMID 11593426.
- ↑ Srivastava DK, Husain I, Arteaga CL, Wilson SH (June 1999). "DNA polymerase beta expression differences in selected human tumors and cell lines". Carcinogenesis 20 (6): 1049–54. doi:10.1093/carcin/20.6.1049. PMID 10357787.
- ↑ He F, Yang XP, Srivastava DK, Wilson SH (January 2003). "DNA polymerase beta gene expression: the promoter activator CREB-1 is upregulated in Chinese hamster ovary cells by DNA alkylating agent-induced stress". Biol. Chem. 384 (1): 19–23. doi:10.1515/BC.2003.003. PMID 12674496.
- ↑ Narayan S, He F, Wilson SH (August 1996). "Activation of the human DNA polymerase beta promoter by a DNA-alkylating agent through induced phosphorylation of cAMP response element-binding protein-1". J. Biol. Chem. 271 (31): 18508–13. doi:10.1074/jbc.271.31.18508. PMID 8702497.
- ↑ Sarnowska E; Grzybowska EA; Sobczak K et al. (2007). "Hairpin structure within the 3'UTR of DNA polymerase beta mRNA acts as a post-transcriptional regulatory element and interacts with Hax-1". Nucleic Acids Res. 35 (16): 5499–510. doi:10.1093/nar/gkm502. PMC 2018635. PMID 17704138.
- ↑ Whitehouse, C J; Taylor R M; Thistlethwaite A; Zhang H; Karimi-Busheri F; Lasko D D; Weinfeld M; Caldecott K W (Jan 2001). "XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair". Cell (United States) 104 (1): 107–17. doi:10.1016/S0092-8674(01)00195-7. ISSN 0092-8674. PMID 11163244.
- ↑ Wang, Liming; Bhattacharyya Nandan; Chelsea Diane M; Escobar Pedro F; Banerjee Sipra (Nov 2004). "A novel nuclear protein, MGC5306 interacts with DNA polymerase beta and has a potential role in cellular phenotype". Cancer Res. (United States) 64 (21): 7673–7. doi:10.1158/0008-5472.CAN-04-2801. ISSN 0008-5472. PMID 15520167.
- ↑ Fan, Jinshui; Otterlei Marit; Wong Heng-Kuan; Tomkinson Alan E; Wilson David M (2004). "XRCC1 co-localizes and physically interacts with PCNA". Nucleic Acids Res. (England) 32 (7): 2193–201. doi:10.1093/nar/gkh556. PMC 407833. PMID 15107487.
- ↑ Kubota, Y; Nash R A; Klungland A; Schär P; Barnes D E; Lindahl T (Dec 1996). "Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein". EMBO J. (ENGLAND) 15 (23): 6662–70. ISSN 0261-4189. PMC 452490. PMID 8978692.
- ↑ Bhattacharyya, N; Banerjee S (Jul 2001). "A novel role of XRCC1 in the functions of a DNA polymerase beta variant". Biochemistry (United States) 40 (30): 9005–13. doi:10.1021/bi0028789. ISSN 0006-2960. PMID 11467963.
Further reading
- Date T; Tanihara K; Yamamoto S et al. (1992). "Two regions in human DNA polymerase beta mRNA suppress translation in Escherichia coli". Nucleic Acids Res. 20 (18): 4859–64. doi:10.1093/nar/20.18.4859. PMC 334243. PMID 1408801.
- Wang L, Patel U, Ghosh L, Banerjee S (1992). "DNA polymerase beta mutations in human colorectal cancer". Cancer Res. 52 (17): 4824–7. PMID 1511447.
- Tokui T; Inagaki M; Nishizawa K et al. (1991). "Inactivation of DNA polymerase beta by in vitro phosphorylation with protein kinase C". J. Biol. Chem. 266 (17): 10820–4. PMID 2040602.
- SenGupta DN; Zmudzka BZ; Kumar P et al. (1986). "Sequence of human DNA polymerase beta mRNA obtained through cDNA cloning". Biochem. Biophys. Res. Commun. 136 (1): 341–7. doi:10.1016/0006-291X(86)90916-2. PMID 2423078.
- Zmudzka BZ, Fornace A, Collins J, Wilson SH (1988). "Characterization of DNA polymerase beta mRNA: cell-cycle and growth response in cultured human cells". Nucleic Acids Res. 16 (20): 9587–96. doi:10.1093/nar/16.20.9587. PMC 338765. PMID 2460824.
- Widen SG, Kedar P, Wilson SH (1988). "Human beta-polymerase gene. Structure of the 5'-flanking region and active promoter". J. Biol. Chem. 263 (32): 16992–8. PMID 3182828.
- Abbotts J; SenGupta DN; Zmudzka B et al. (1988). "Expression of human DNA polymerase beta in Escherichia coli and characterization of the recombinant enzyme". Biochemistry 27 (3): 901–9. doi:10.1021/bi00403a010. PMID 3284575.
- Dobashi Y; Kubota Y; Shuin T et al. (1995). "Polymorphisms in the human DNA polymerase beta gene". Hum. Genet. 95 (4): 389–90. doi:10.1007/bf00208961. PMID 7705833.
- Chyan YJ; Ackerman S; Shepherd NS et al. (1994). "The human DNA polymerase beta gene structure. Evidence of alternative splicing in gene expression". Nucleic Acids Res. 22 (14): 2719–25. doi:10.1093/nar/22.14.2719. PMC 308239. PMID 7914364.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Chang M; Burmer GC; Sweasy J et al. (1994). "Evidence against DNA polymerase beta as a candidate gene for Werner syndrome". Hum. Genet. 93 (5): 507–12. doi:10.1007/bf00202813. PMID 8168825.
- Chyan YJ, Strauss PR, Wood TG, Wilson SH (1996). "Identification of novel mRNA isoforms for human DNA polymerase beta". DNA Cell Biol. 15 (8): 653–9. doi:10.1089/dna.1996.15.653. PMID 8769567.
- Pelletier H; Sawaya MR; Wolfle W et al. (1996). "Crystal structures of human DNA polymerase beta complexed with DNA: implications for catalytic mechanism, processivity, and fidelity". Biochemistry 35 (39): 12742–61. doi:10.1021/bi952955d. PMID 8841118.
- Pelletier H; Sawaya MR; Wolfle W et al. (1996). "A structural basis for metal ion mutagenicity and nucleotide selectivity in human DNA polymerase beta". Biochemistry 35 (39): 12762–77. doi:10.1021/bi9529566. PMID 8841119.
- Pelletier H, Sawaya MR (1996). "Characterization of the metal ion binding helix-hairpin-helix motifs in human DNA polymerase beta by X-ray structural analysis". Biochemistry 35 (39): 12778–87. doi:10.1021/bi960790i. PMID 8841120.
- Kubota Y; Nash RA; Klungland A et al. (1997). "Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein". EMBO J. 15 (23): 6662–70. PMC 452490. PMID 8978692.
- Bennett RA, Wilson DM, Wong D, Demple B (1997). "Interaction of human apurinic endonuclease and DNA polymerase beta in the base excision repair pathway". Proc. Natl. Acad. Sci. U.S.A. 94 (14): 7166–9. doi:10.1073/pnas.94.14.7166. PMC 23779. PMID 9207062.
- Sawaya MR; Prasad R; Wilson SH et al. (1997). "Crystal structures of human DNA polymerase beta complexed with gapped and nicked DNA: evidence for an induced fit mechanism". Biochemistry 36 (37): 11205–15. doi:10.1021/bi9703812. PMID 9287163.
- Bhattacharyya N, Banerjee S (1997). "A variant of DNA polymerase beta acts as a dominant negative mutant". Proc. Natl. Acad. Sci. U.S.A. 94 (19): 10324–9. doi:10.1073/pnas.94.19.10324. PMC 23361. PMID 9294209.
- Suzuki Y; Yoshitomo-Nakagawa K; Maruyama K et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Tan, X. H.; Zhao, M.; Pan, K. F.; Dong, Y.; Dong, B.; Feng, G. J.; Jia, G.; Lu, Y. Y. (2005). "Frequent mutation related with overexpression of DNA polymerase beta in primary tumors and precancerous lesions of human stomach". Cancer Letters 220 (1): 101–114. doi:10.1016/j.canlet.2004.07.049. PMID 15737693.
External links
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||