DNA-binding protein



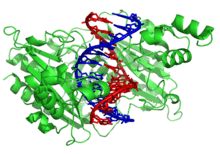
DNA-binding proteins [3][4][5] are proteins that are composed of DNA-binding domains and thus have a specific or general affinity for either single or double stranded DNA. Sequence-specific DNA-binding proteins generally interact with the major groove of B-DNA, because it exposes more functional groups that identify a base pair. However there are some known minor groove DNA-binding ligands such as netropsin,[6] distamycin, Hoechst 33258, pentamidine, DAPI and others.[7]
Examples
DNA-binding proteins include transcription factors which modulate the process of transcription, various polymerases, nucleases which cleave DNA molecules, and histones which are involved in chromosome packaging and transcription in the cell nucleus. DNA-binding proteins can incorporate such domains as the zinc finger, the helix-turn-helix, and the leucine zipper (among many others) that facilitate binding to nucleic acid. there are also more unusual examples such as transcription activator like effectors.
Non-specific DNA-protein interactions
Structural proteins that bind DNA are well-understood examples of non-specific DNA-protein interactions. Within chromosomes, DNA is held in complexes with structural proteins. These proteins organize the DNA into a compact structure called chromatin. In eukaryotes this structure involves DNA binding to a complex of small basic proteins called histones, while in prokaryotes multiple types of proteins are involved.[8][9] The histones form a disk-shaped complex called a nucleosome, which contains two complete turns of double-stranded DNA wrapped around its surface. These non-specific interactions are formed through basic residues in the histones making ionic bonds to the acidic sugar-phosphate backbone of the DNA, and are therefore largely independent of the base sequence.[10] Chemical modifications of these basic amino acid residues include methylation, phosphorylation and acetylation.[11] These chemical changes alter the strength of the interaction between the DNA and the histones, making the DNA more or less accessible to transcription factors and changing the rate of transcription.[12] Other non-specific DNA-binding proteins in chromatin include the high-mobility group proteins, which bind to bent or distorted DNA.[13] These proteins are important in bending arrays of nucleosomes and arranging them into the larger structures that make up chromosomes.[14]
DNA-binding proteins that specifically bind single-stranded DNA
(See Single-stranded binding protein) A distinct group of DNA-binding proteins are the DNA-binding proteins that specifically bind single-stranded DNA. In humans, replication protein A is the best-understood member of this family and is used in processes where the double helix is separated, including DNA replication, recombination and DNA repair.[15] These binding proteins seem to stabilize single-stranded DNA and protect it from forming stem-loops or being degraded by nucleases.
Binding to particular DNA sequences
In contrast, other proteins have evolved to bind to particular DNA sequences. The most intensively studied of these are the various transcription factors, which are proteins that regulate transcription. Each transcription factor binds to one particular set of DNA sequences and activates or inhibits the transcription of genes that have these sequences close to their promoters. The transcription factors do this in two ways. Firstly, they can bind the RNA polymerase responsible for transcription, either directly or through other mediator proteins; this locates the polymerase at the promoter and allows it to begin transcription.[16] Alternatively, transcription factors can bind enzymes that modify the histones at the promoter; this will change the accessibility of the DNA template to the polymerase.[17]
As these DNA targets can occur throughout an organism's genome, changes in the activity of one type of transcription factor can affect thousands of genes.[18] Consequently, these proteins are often the targets of the signal transduction processes that control responses to environmental changes or cellular differentiation and development. The specificity of these transcription factors' interactions with DNA come from the proteins making multiple contacts to the edges of the DNA bases, allowing them to "read" the DNA sequence. Most of these base-interactions are made in the major groove, where the bases are most accessible.[19] Mathematical descriptions of protein-DNA binding taking into account sequence-specificity, competitive and cooperative binding of proteins of different types are usually performed with the help of the lattice models.[20] Computational methods to identify the DNA binding sequence specificity have been proposed to make a good use of the abundant sequence data in the post-genomic era.[21]
See also
- bZIP domain
- DNA-binding domain
- Helix-loop-helix
- Helix-turn-helix
- HMG-box
- Leucine zipper
- Lexitropsin
- Nucleic acid simulations
- Single-strand binding protein
- Zinc finger
References
- ↑ Created from PDB 1LMB
- ↑ Created from PDB 1RVA
- ↑ Travers, A. A. (1993). DNA-protein interactions. London: Springer. ISBN 978-0-412-25990-6.
- ↑ Pabo CO, Sauer RT (1984). "Protein-DNA recognition". Annu. Rev. Biochem. 53 (1): 293–321. doi:10.1146/annurev.bi.53.070184.001453. PMID 6236744.
- ↑ Dickerson R.E. (1983). "The DNA helix and how it is read". Sci Am 249 (6): 94–111. doi:10.1038/scientificamerican1283-94.
- ↑ Zimmer C, Wähnert U (1986). "Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material". Prog. Biophys. Mol. Biol. 47 (1): 31–112. doi:10.1016/0079-6107(86)90005-2. PMID 2422697.
- ↑ Dervan PB (April 1986). "Design of sequence-specific DNA-binding molecules". Science 232 (4749): 464–71. doi:10.1126/science.2421408. PMID 2421408.
- ↑ Sandman K, Pereira S, Reeve J (1998). "Diversity of prokaryotic chromosomal proteins and the origin of the nucleosome". Cell Mol Life Sci 54 (12): 1350–64. doi:10.1007/s000180050259. PMID 9893710.
- ↑ Dame RT (2005). "The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin". Mol. Microbiol. 56 (4): 858–70. doi:10.1111/j.1365-2958.2005.04598.x. PMID 15853876.
- ↑ Luger K, Mäder A, Richmond R, Sargent D, Richmond T (1997). "Crystal structure of the nucleosome core particle at 2.8 A resolution". Nature 389 (6648): 251–60. doi:10.1038/38444. PMID 9305837.
- ↑ Jenuwein T, Allis C (2001). "Translating the histone code". Science 293 (5532): 1074–80. doi:10.1126/science.1063127. PMID 11498575.
- ↑ Ito T (2003). "Nucleosome assembly and remodelling". Curr Top Microbiol Immunol 274: 1–22. doi:10.1007/978-3-642-55747-7_1. PMID 12596902.
- ↑ Thomas J (2001). "HMG1 and 2: architectural DNA-binding proteins". Biochem Soc Trans 29 (Pt 4): 395–401. doi:10.1042/BST0290395. PMID 11497996.
- ↑ Grosschedl R, Giese K, Pagel J (1994). "HMG domain proteins: architectural elements in the assembly of nucleoprotein structures". Trends Genet 10 (3): 94–100. doi:10.1016/0168-9525(94)90232-1. PMID 8178371.
- ↑ Iftode C, Daniely Y, Borowiec J (1999). "Replication protein A (RPA): the eukaryotic SSB". Crit Rev Biochem Mol Biol 34 (3): 141–80. doi:10.1080/10409239991209255. PMID 10473346.
- ↑ Myers L, Kornberg R (2000). "Mediator of transcriptional regulation". Annu Rev Biochem 69 (1): 729–49. doi:10.1146/annurev.biochem.69.1.729. PMID 10966474.
- ↑ Spiegelman B, Heinrich R (2004). "Biological control throughs regulated transcriptional coactivators". Cell 119 (2): 157–67. doi:10.1016/j.cell.2004.09.037. PMID 15479634.
- ↑ Li Z, Van Calcar S, Qu C, Cavenee W, Zhang M, Ren B (2003). "A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells". Proc Natl Acad Sci USA 100 (14): 8164–9. doi:10.1073/pnas.1332764100. PMC 166200. PMID 12808131.
- ↑ Pabo C, Sauer R (1984). "Protein-DNA recognition". Annu Rev Biochem 53 (1): 293–321. doi:10.1146/annurev.bi.53.070184.001453. PMID 6236744.
- ↑ Teif V.B., Rippe K. (2010). "Statistical-mechanical lattice models for protein-DNA binding in chromatin.". Journal of Physics: Condensed Matter 22 (41): 414105. arXiv:1004.5514. doi:10.1088/0953-8984/22/41/414105. PMID 21386588.
- ↑ Wong KC, Chan TM, Peng C., Li Y., and Zhang Z. "DNA Motif Elucidation using belief propagation" Nucleic Acids Research Advanced Online June 2013; doi:10.1093/nar/gkt574 PMID 23814189
External links
- Abalone tool for modeling DNA-ligand interactions.
- DBD database of predicted transcription factors Uses a curated set of DNA-binding domains to predict transcription factors in all completely sequenced genomes
- DNA-Binding Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||||||||||||||||