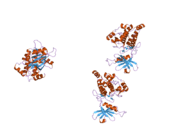DAPK2
Death-associated protein kinase 2 is an enzyme that in humans is encoded by the DAPK2 gene.[1][2]
This gene encodes a protein that belongs to the serine/threonine protein kinase family. This protein contains a N-terminal protein kinase domain followed by a conserved calmodulin-binding domain with significant similarity to that of death-associated protein kinase 1 (DAPK1), a positive regulator of programmed cell death. Overexpression of this gene was shown to induce cell apoptosis. It uses multiple polyadenylation sites.[2] The DAPK2 mRNA may undergo alternative splicing to produce a DAPK3-like encoding transcript. [3]
References
Further reading
- Inbal B, Shani G, Cohen O et al. (2000). "Death-Associated Protein Kinase-Related Protein 1, a Novel Serine/Threonine Kinase Involved in Apoptosis". Mol. Cell. Biol. 20 (3): 1044–54. doi:10.1128/MCB.20.3.1044-1054.2000. PMC 85221. PMID 10629061.
- Shani G, Henis-Korenblit S, Jona G et al. (2001). "Autophosphorylation restrains the apoptotic activity of DRP-1 kinase by controlling dimerization and calmodulin binding". EMBO J. 20 (5): 1099–113. doi:10.1093/emboj/20.5.1099. PMC 145456. PMID 11230133.
- Wong TS, Chang HW, Tang KC et al. (2002). "High frequency of promoter hypermethylation of the death-associated protein-kinase gene in nasopharyngeal carcinoma and its detection in the peripheral blood of patients". Clin. Cancer Res. 8 (2): 433–7. PMID 11839660.
- Chan MW, Chan LW, Tang NL et al. (2002). "Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients". Clin. Cancer Res. 8 (2): 464–70. PMID 11839665.
- Inbal B, Bialik S, Sabanay I et al. (2002). "DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death". J. Cell Biol. 157 (3): 455–68. doi:10.1083/jcb.200109094. PMC 2173279. PMID 11980920.
- Satoh A, Toyota M, Itoh F et al. (2002). "DNA methylation and histone deacetylation associated with silencing DAP kinase gene expression in colorectal and gastric cancers". Br. J. Cancer 86 (11): 1817–23. doi:10.1038/sj.bjc.6600319. PMC 2375414. PMID 12087472.
- Strausberg RL, Feingold EA, Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Gerhard DS, Wagner L, Feingold EA et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Barrios-Rodiles M, Brown KR, Ozdamar B et al. (2005). "High-throughput mapping of a dynamic signaling network in mammalian cells". Science 307 (5715): 1621–5. doi:10.1126/science.1105776. PMID 15761153.
- Olsen JV, Blagoev B, Gnad F et al. (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
- Rizzi M, Tschan MP, Britschgi C et al. (2007). "The death-associated protein kinase 2 is up-regulated during normal myeloid differentiation and enhances neutrophil maturation in myeloid leukemic cells". J. Leukoc. Biol. 81 (6): 1599–608. doi:10.1189/jlb.0606400. PMID 17347302.
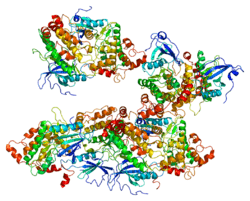
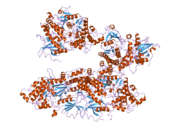
PDB gallery |
|---|
| | 1wmk: Human death-associated kinase DRP-1, mutant S308D d40 |
| 1z9x: Human DRP-1 kinase, W305S S308A D40 mutant, crystal form with 3 monomers in the asymmetric unit |
| 1zws: Crystal structure of the catalytic domain of human DRP-1 kinase |
| 2a27: Human DRP-1 kinase, W305S S308A D40 mutant, crystal form with 8 monomers in the asymmetric unit |
| 2a2a: High-resolution crystallographic analysis of the autoinhibited conformation of a human death-associated protein kinase |
| 2cke: HUMAN DEATH-ASSOCIATED DRP-1 KINASE IN COMPLEX WITH INHIBITOR |
|
|
|
|
|---|
| | | | | | |
- Biochemistry overview
- Enzymes overview
- By EC number: 1.1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 10
- 11
- 13
- 14
- 15-18
- 2.1
- 3.1
- 4.1
- 5.1
- 6.1-3
|
|
|
|