D-lactate dehydrogenase (cytochrome)
| D-lactate dehydrogenase (cytochrome) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.1.2.4 | ||||||||
| CAS number | 37250-79-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a D-lactate dehydrogenase (cytochrome) (EC 1.1.2.4) is an enzyme that catalyzes the chemical reaction
- (D)-lactate + 2 ferricytochrome c
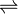 pyruvate + 2 ferrocytochrome c
pyruvate + 2 ferrocytochrome c
Thus, the two substrates of this enzyme are (D)-lactate and ferricytochrome c, whereas its two products are pyruvate and ferrocytochrome c.
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with a cytochrome as acceptor. The systematic name of this enzyme class is (D)-lactate:ferricytochrome-c 2-oxidoreductase. Other names in common use include lactic acid dehydrogenase, D-lactate (cytochrome) dehydrogenase, cytochrome-dependent D-(−)-lactate dehydrogenase, D-lactate-cytochrome c reductase, and D-(−)-lactic cytochrome c reductase. This enzyme participates in pyruvate metabolism. It employs one cofactor, FAD. This type of enzyme has been characterized in animals, fungi, bacteria and recently in plants[1] .[2] It is believed to be important in the detoxification of methylglyoxal through the glyoxylase pathway
References
- ↑ Atlante, A., de Bari, L., Valenti, D., Pizzuto, R., Paventi, G., and Passarella, S. (2005). "Transport and metabolism of D-lactate in Jerusalem artichoke mitochondria". Biochim. Biophys. Acta 1708 (1): 13–22. doi:10.1016/j.bbabio.2005.03.003. PMID 15949980.
- ↑ Martin Engqvist, Maria Fabiana Drincovich, Ulf-Ingo Flügge, and Veronica G. Maurino (2009). "Two D-2-hydroxyacid dehydrogenases in Arabidopsis thaliana with catalytic capacities to participate in the last reactions of the methylglyoxal and {beta}-oxidation pathways.". J Biol Chem. 284 (September 11): 25026–25037. doi:10.1074/jbc.M109.021253. PMC 2757207. PMID 19586914.
- GREGOLIN C, SINGER TP (1963). "The lactic dehydrogenase of yeast. III. D(-)Lactic cytochrome c reductase, a zinc-flavoprotein from aerobic yeast". Biochim. Biophys. Acta. 67: 201–18. doi:10.1016/0006-3002(63)91818-3. PMID 13950255.
- GREGOLIN C, SINGER TP, KEARNEY EB, BOERI E (1961). "The formation and enzymatic properties of the various lactic dehydrogenases of yeast". Ann. N. Y. Acad. Sci. 94 (3): 780–97. doi:10.1111/j.1749-6632.1961.tb35573.x. PMID 13901630.
- Nygaard AP (1961). "D(−)-Lactate cytochrome c reductase, a flavoprotein from yeast". J. Biol. Chem. 236: 920–925.
- Boyer, P.D., Lardy, H. and Myrback, K. (Eds.), The Enzymes, 2nd ed., vol. 7, Academic Press, New York, 1963, p. 557-565.