Cyclopentenone
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Cyclopenten-1-one | |||
| Identifiers | |||
| 930-30-3 | |||
| ChEMBL | ChEMBL52190 | ||
| ChemSpider | 12999 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 13588 | ||
| |||
| Properties | |||
| C5H6O | |||
| Molar mass | 82.04 g·mol−1 | ||
| Density | 0.98 g·mL−1 | ||
| Boiling point | 150 °C (302 °F; 423 K) | ||
| almost insoluble in water | |||
| Hazards | |||
| Main hazards | Harmful | ||
| Flash point | 42 °C (108 °F; 315 K) | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
2-Cyclopentenone is a hydrocarbon with chemical formula C5H6O and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. 2-Cyclopentenone belongs to the cycloalkene (alkenes which have one or more rings of carbon atoms) class of compounds and is also a ketone (it possesses a carbonyl functional group). It is a liquid at room temperature with a boiling point of 150 °C. It has been isolated from pressure-cooked pork liver by simultaneous steam distillation and continuous solvent extraction.[1]
The term cyclopentenone may also refer to a structural motif wherein the cyclopentenone moiety is a subsection of a larger molecule. In this regard, cyclopentenones are found in a large number of natural products, including jasmone, the aflatoxins, and several prostaglandins.
Synthesis
2-Cyclopentenones can be synthesized in a number of ways. Industrially, the most common procedures are elimination of α-bromo-cyclopentanone using lithium carbonate[2] and Claisen condensation-decarboxylation-isomerization cascades of unsaturated diesters as shown below.[3]
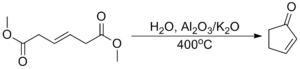
As a functional group, the synthesis of 2-cyclopentenones is accomplished in a variety of other ways, including the Nazarov cyclization reaction from divinyl ketones, Saegusa–Ito oxidation from cyclopentanones, ring-closing metathesis from the corresponding dienes, oxidation of the corresponding cyclic allylic alcohols, and the Pauson–Khand reaction from alkenes, alkynes, and carbon monoxide.[4]
Reactions
As an enone, 2-cyclopentenone undergoes the typical reactions of α-β unsaturated ketones, including nucleophilic conjugate addition, the Baylis–Hillman reaction, and the Michael reaction. Cyclopentenone also functions as an excellent dienophile in the Diels–Alder reaction, reacting with a wide variety of dienes. In one example, a Danishefsky-type diene is reacted with a cyclopentenone to yield a fused tricyclic system en route to the synthesis of coriolin.[5]
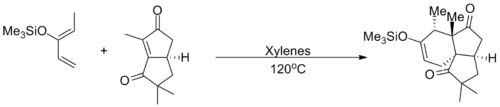
References
- ↑ Mussinan, Cynthia J.; Walradt, John P. (May 1974). "Volatile constituents of pressure cooked pork liver". Journal of Agricultural and Food Chemistry 22 (5): 827–831. doi:10.1021/jf60195a002.
- ↑ US EP1418166, Daisuke, Fukushima & Hirata Norihiko, "Process for producing 2-bromocyclopentanone", published 2004-05-12
- ↑ US EP1422212, Liang, Shelue; Andrea Haunert & Sylvia Huber-Dirr et al., "Process for preparing cyclopentenone", published 2004-11-25
- ↑ Müller, Reto. "Synthesis of cyclopentenones". Organic Chemistry Portal. Retrieved 3 March 2015.
- ↑ Danishefsky, Samuel; Zamboni, Robert; Kahn, Michael; Etheredge, Sarah Jane (March 1980). "Total synthesis of dl-coriolin". Journal of the American Chemical Society 102 (6): 2097–2098. doi:10.1021/ja00526a061.