Cyclopamine
 | |
| Names | |
|---|---|
| IUPAC name
(3β,23R)-17,23-Epoxyveratraman-3-ol | |
| Other names
• 11-Deoxojervine • (2′R,3S,3′R,3′aS,6′S,6aS,6bS,7′aR,11aS,11bR)-1,2,3,3′a,4,4′,5′,6,6′,6a,6b,7,7′,7'a,8,11,11a,11b-octadecahydro-3′,6′,10,11b-tetramethyl-spiro[9H-benzo[a]fluorene-9,2′(3′H)-furo[3,2-b]pyridin]-3-ol | |
| Identifiers | |
| 4449-51-8 | |
| ChEMBL | ChEMBL254129 |
| ChemSpider | 391275 |
| |
| Jmol-3D images | Image |
| PubChem | 442972 |
| |
| UNII | ZH658AJ192 |
| Properties | |
| Molecular formula |
C27H41NO2 |
| Molar mass | 411.62 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
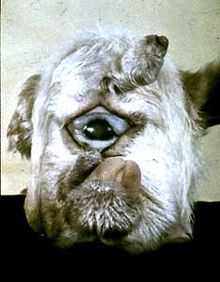
Cyclopamine (11-deoxojervine) is a naturally occurring chemical that belongs to the group of steroidal jerveratrum alkaloids. It is a teratogen isolated from the corn lily (Veratrum californicum) that causes usually fatal birth defects. It can prevent the fetal brain from dividing into two lobes (holoprosencephaly) and cause the development of a single eye (cyclopia). It does so by inhibiting the hedgehog signaling pathway (Hh). Cyclopamine is useful in studying the role of Hh in normal development, and as a potential treatment for certain cancers in which Hh is overexpressed.
Cyclopamine was named for one-eyed lambs which were born to sheep which grazed on wild corn lily at a farm in Idaho. In 1957 the US Department of Agriculture started an eleven-year investigation which led to the identification of cyclopamine as the cause of the birth defect.[1]
Physiological effects
Cyclopamine inhibits the hedgehog signaling pathway (Hh) by influencing the balance between the active and inactive forms of the smoothened protein.
Medical potential
Cyclopamine is currently being investigated as a treatment agent in basal cell carcinoma, medulloblastoma, and rhabdomyosarcoma, tumors that result from excessive Hh activity,[2] glioblastoma, and as a treatment agent for multiple myeloma.
Studies suggest that cyclopamine acts as a primary inhibitor of the hedgehog signaling pathway in cells. This pathway named for the ligand for the signal protein, is used by cells to help them react to external chemical signals. The pathway carries out important functions in embryonic development and when it goes awry, deformities can occur. However, errant activation of the pathway can also trigger cancer in adult humans, leading to basal cell carcinoma, medulloblastoma, rhabdomyosarcoma, and prostate, pancreatic and breast cancers. A way of controlling the pathway using cyclopamine could turn this problem on its head and provide a way to treat cancer. Many anticancer drugs are paradoxically carcinogenic in healthy individuals.[3]
The cyclopamine derivative IPI-926 is in clinical trials for the treatment of various types of cancer.[4]
See also
- Saridegib (also known as IPI-926), a semi-synthetic analog of cyclopamine, a natural Hh pathway inhibitor
- Vismodegib, an artificial Hh signaling inhibitor
- Sonidegib, an artificial Hh signaling inhibitor
References
- ↑ Herper, Matthew (2005-11-28). "The Curious Case of The One-Eyed Sheep". Forbes.
- ↑ Beachy, Philip A.; Taipale, Jussi; Chen, James K.; Cooper, Michael K.; Wang, Baolin; Mann, Randall K.; Milenkovic, Ljiljana; Scott, Matthew P. (2000-08-31). "Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine". Nature 406 (6799): 1005–9. doi:10.1038/35023008. PMID 10984056.
- ↑ David Bradley (2009-08-15). "Keeping an eye on anticancer drug". NMR Knowledge Base.
- ↑ "Pipeline: IPI-926". Infinity Pharmaceuticals.
External links
- Cancer Drug Behind Cyclops Birth?, Wired News
- Experimental Anti-cancer Drug Kills Brain Tumor Stem Cells, Science Daily
- Tabs, S; Avci, O (2004). "Induction of the differentiation and apoptosis of tumor cells in vivo with efficiency and selectivity". European Journal of Dermatology 14 (2): 96–102. PMID 15196999.
- Taş, S; Avci, O (2004). "Rapid clearance of psoriatic skin lesions induced by topical cyclopamine. A preliminary proof of concept study". Dermatology (Basel, Switzerland) 209 (2): 126–31. doi:10.1159/000079596. PMID 15316166.
- Zhang, J; Garrossian, M; Gardner, D; Garrossian, A; Chang, YT; Kim, YK; Chang, CW (2008). "Synthesis and anticancer activity studies of cyclopamine derivatives". Bioorganic & Medicinal Chemistry Letters 18 (4): 1359–63. doi:10.1016/j.bmcl.2008.01.017. PMID 18221872.
- Fan, Q; Gu, D; He, M; Liu, H; Sheng, T; Xie, G; Li, C. X.; Zhang, X; Wainwright, B; Garrossian, A; Garrossian, M; Gardner, D; Xie, J (2011). "Tumor shrinkage by cyclopamine tartrate through inhibiting hedgehog signaling". Chinese Journal of Cancer 30 (7): 472–81. doi:10.5732/cjc.011.10157. PMC 4013422. PMID 21718593.