Cyclooctadiene iridium chloride dimer
 | |
| Names | |
|---|---|
| Other names
Bis(1,5-cyclooctadiene)diiridium(I) dichloride | |
| Identifiers | |
| 12112-67-3 | |
| Properties | |
| C16H24Cl2Ir2 | |
| Molar mass | 671.70 |
| Appearance | red-orange solid |
| Density | 2.65 g/cm3 (red polymorph) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
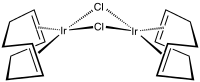
Cyclooctadiene iridium chloride dimer is an organoiridium compound with the formula Ir2Cl2(C8H12)2, where C8H12 is the diene 1,5-cyclooctadiene. It is an orange solid that is soluble in organic solvents. The complex is used as a precursor to other iridium complexes, some of which are used in homogeneous catalysis.[1] The solid is air-stable but its solutions degrades in air.
Preparation, structure, reactions
The compound is prepared by heating hydrated iridium trichloride and cyclooctadiene in alcohol solvent. In the process, Ir(III) is reduced to Ir(I).[2]
In terms of its molecular structure, the iridium centers are square planar as is typical for a d8 complex. The Ir2Cl2 core is folded with a dihedral angle of 86°. The molecule crystallizes in yellow-orange and red-orange polymorphs; the latter one is more common.[3][4]
The complex is widely used precursor to other iridium complexes. A notable derivative is Crabtree's catalyst.[5] The chloride ligands can also be replaced to give diiridium complexes exhibiting modified reactivity, e.g. Ir2(OCH3)2(C8H12)2. A closely related but still more reactive complex is chlorobis(cyclooctene)iridium dimer.
See also
References
- ↑ J. Hartwig, "Organotransition Metal Chemistry: From Bonding to Catalysis" University Science Books, 2009. ISBN 978-1891389535.
- ↑ J. L. Herdé, J. C. Lambert, C. V. Senoff "Cyclooctene and 1,5-Cyclooctadiene Complexes of Iridium(I)" Inorganic Syntheses, 1974, Volume 15, pp. 18–20. doi:10.1002/9780470132463.ch5
- ↑ F.Albert Cotton, Pascual Lahuerta, Mercedes Sanau, Willi Schwotzer "Air oxidation of Ir2(Cl)2(COD)2 revisited. The structures of [Ir(μ2-Cl)(COD)]2 (ruby form) and its oxidation product, Ir2Cl2(COD)2(μ2-OH)2(μ2-O)" Inorganica Chimica Acta, 1986 vol. 120, Pages 153–157. doi:10.1016/S0020-1693(00)86102-2
- ↑ Tabrizi, D., Manoli, J. M., Dereigne, A., "Etude radiocristallographique de μ-dichloro-bis (π cyclooctadiène-1,5) diiridium: [(COD-1,5)IrCl]2, variété jaune-orange", Journal of the Less Common Metals 1970, vol. 21, pp. 337. doi:10.1016/0022-5088(70)90155-4
- ↑ Robert H. Crabtree, Sheila M. Morehouse "[η4-1,5-Cyclooctadiene)(Pyridine)-(Tricyclohexylphosphine)Iridium(I)Hexafluorophosphate" Inorganic Syntheses, 1986, Volume 24, pp. 173–176. doi:10.1002/9780470132555.ch50
| ||||||||||||||||||||||||||||||||||||||||