Cyclomethicone
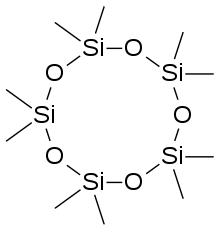
Cyclomethicone is a volatile methyl siloxane which makes up a class of liquid silicones (cyclic polydimethylsiloxane polymers) that possess the characteristics of low viscosity and high volatility as well as being skin emollients and in certain circumstances useful cleaning solvents.[1] Unlike dimethicones, which are linear siloxanes that do not evaporate, cyclomethicones are cyclic: both groups consist of a polymer featuring a monomer backbone of one silicon and two oxygen atoms bonded together, but instead of having a very long "linear" backbone surrounded by a series of methyl groups (which produces a clear, non-reactive, non-volatile liquid ranging from water-thin to taffy-thick), cyclomethicones have short backbones that make closed or nearly-closed rings or "cycles" with their methyl groups, giving them many of the same properties of dimethicones but making them much more volatile. They are used in many cosmetic products where eventual complete evaporation of the siloxane carrier fluid is desired. In this way they are useful for products like deodorants and antiperspirants which need to coat the skin but not remain tacky afterward.[2] Most cyclomethicone is manufactured by Dow Corning.[3]
Nomenclature
| Cyclic siloxanes (cyclomethicones) | CAS |
|---|---|
| D3: hexamethylcyclotrisiloxane | 541-05-9 |
| D4: octamethylcyclotetrasiloxane | 556-67-2 |
| D5: decamethylcyclopentasiloxane | 541-02-6 |
| D6: dodecamethylcyclohexasiloxane | 540-97-6 |
Safety and environmental considerations
Because silicones are heavily used in biomedical and cosmetic applications, their toxicology has been intensively examined. "The inertness of silicones toward warmblooded animals has been demonstrated in a number of tests." With an LD50 in rats of>50 g/kg, they are virtually nontoxic.[4]
Cyclomethicones are ubiquitous[5] because they are widely used in biomedical and cosmetic applications. and can be found at high levels in American cities.[6] The cyclomethicone D4 is bioaccumulative and can be toxic to aquatic animals in concentrations found in the environment.[7] D4 can cause liver damage, has an estrogenic effect and impairs fertility in mammals.[7]
In the European Union, D4 and D5 have been deemed hazardous as per the REACH directive. D4 is regulated as a pollutant in Canada.[5]
References
- ↑ Barbara Kanegsberg; Edward Kanegsberg (2011). Handbook for Critical Cleaning: Cleaning agents and systems. CRC. p. 19. ISBN 978-1-4398-2827-4.
- ↑ Amarjit Sahota (25 November 2013). Sustainability: how the cosmetics industry is greening up. Wiley. p. 208. ISBN 978-1-118-67650-9.
- ↑ Meyer Rosen (23 September 2005). Delivery System Handbook for Personal Care and Cosmetic Products: Technology, Applications and Formulations. William Andrew. p. 693. ISBN 978-0-8155-1682-8.
- ↑ Moretto, Hans-Heinrich; Schulze, Manfred; Wagner, Gebhard (2005). "Silicones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_057.
- ↑ 5.0 5.1 Karpus, Jennifer (20 June 2014). "Exec: Silicone industry must focus on safety, environment". Rubber & Plastic News. Retrieved 8 April 2015.
- ↑ Bienkowski, Brian (30 April 2013). "Chemicals from Personal Care Products Pervasive in Chicago Air". Scientific American. Retrieved 8 April 2015.
- ↑ 7.0 7.1 Wang, De-Gao; Norwood, Warren; Alaee, Mehran; Byer, Jonatan D.; Brimble, Samantha (October 2013). "Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment". Chemosphere 93 (5): 711–725. doi:10.1016/j.chemosphere.2012.10.041.