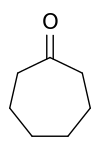
Cycloheptanone
 | |
| Names | |
|---|---|
| IUPAC name
Cycloheptanone | |
| Other names
Suberone | |
| Identifiers | |
| 502-42-1 | |
| ChEMBL | ChEMBL18607 |
| ChemSpider | 9971 |
| |
| Jmol-3D images | Image |
| PubChem | 10400 |
| |
| Properties | |
| Molecular formula |
C7H12O |
| Molar mass | 112.17 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.949 g/cm3 (20 °C)[1] |
| Boiling point | 179 to 181 °C (354 to 358 °F; 452 to 454 K)[1] |
| Insoluble | |
| Hazards | |
| R-phrases | R41[2] |
| S-phrases | S23 S24/25 S26 S39[2] |
| Flash point | 56 °C (133 °F; 329 K)[2] |
| Related compounds | |
| Related cyclic ketones |
Cyclohexanone, Cyclooctanone, Tropinone |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Cycloheptanone, (CH2)6CO, is a cyclic ketone also referred to as suberone. It is a colourless volatile liquid. Cycloheptanone is used as a precursor for the synthesis of pharmaceuticals.
Synthesis
In 1836, French chemist Jean-Baptiste Boussingault first synthesized cycloheptanone from the calcium salt of dibasic suberic acid. The destructive distillation of calcium suberate yields calcium carbonate and suberone:[3]
- Ca(O2C(CH2)6CO2) → CaCO3 + (CH2)6CO
Cycloheptanone is still produced by the cyclization and decarboxylation of suberic acid or suberic acid esters. This reaction is typically conducted in the gas phase at 400–450 °C over alumina doped with zinc oxide or cerium oxide.[4]
Cycloheptanone is also produced by the reaction of cyclohexanone with sodium ethoxide and nitromethane. The resulting sodium salt of 1-(nitromethyl)cyclohexanol is added to acetic acid and shaken with hydrogen gas in the presence of W-4 Raney nickel catalyst. Sodium nitrite and acetic acid are then added to give cycloheptanone.[5]
Cycloheptanone is also prepared by ring expansion of cyclohexanone with diazomethane as the methylene source.[5]
Uses and reactions
Cycloheptanone has no direct applications, but is a precursor to other compounds. Bencyclane, a spasmolytic agent and vasodilator is produced from it, for example.[4] Pimelic acid is produced by the oxidative cleavage of cycloheptanone.[6] Dicarboxylic acids such as pimelic acid are useful for the preparation of fragrances and certain polymers.[7]
Several microorganisms, including Mucor plumbeus, Mucor racemosus, and Penicillium chrysogenum, have been found to reduce cycloheptanone to cycloheptanol. These microorganisms have been investigated for use in certain stereospecific enzymatic reactions.[8]
References
- ↑ 1.0 1.1 The Merck Index, 11th Edition, 2728
- ↑ 2.0 2.1 2.2 Cycloheptanone at Sigma-Aldrich
- ↑ Thorpe, T. E. (1912). A Dictionary of Applied Chemistry. LCCN 12009914.
- ↑ 4.0 4.1 Siegel, H.; Eggersdorfer, M. (2005), "Ketones", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a15_077
- ↑ 5.0 5.1 Dauben, H. J. Jr.; Ringold, H. J.; Wade, R. H.; Pearson, D. L.; Anderson, A. G. Jr. (1954). "Cycloheptanone". Org. Synth. 34: 19.; Coll. Vol. 4, p. 221
- ↑ Cornils, B.; Lappe, P. (2005), "Dicarboxylic Acids, Aliphatic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a08_523.pub2
- ↑ "Dicarboxylic Acids". cyberlipids.org.
- ↑ Lemiere, G. L.; Alderweireldt, F. C.; Voets, J. P. (1975). "Reduction of cycloalkanones by several microorganisms". Zeitschrift für Allgemeine Mikrobiologie 15 (2): 89–92. doi:10.1002/jobm.19750150204.