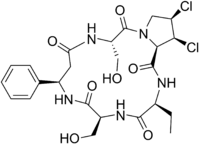
Cyclochlorotine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,2-Dichloro-15-ethyl-5,12-bis-hydroxymethyl-9-phenyl-dodecahydro-3a,6,10,13,16-pentaaza-cyclopentac
yclohexadecene-4,7,11,14,17-pentaone | |
| Other names
Cyclo[(R)-3-phenyl-β-alanyl-L-seryl-(2α,3α,4α)-3,4-dichloro-L-prolyl-L-2-aminobutanoyl-L-seryl]; Yellowed rice toxin | |
| Identifiers | |
| 12663-46-6 | |
| ChemSpider | 28426727 |
| |
| Jmol-3D images | Image |
| KEGG | C19379 |
| PubChem | 25565 |
| |
| Properties | |
| Molecular formula |
C24H31Cl2N5O7 |
| Molar mass | 572.44 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Cyclochlorotine[1] (CC) is a secondary metabolite of the fungus Penicillium islandicum[2] that causes hepatic necrosis and has carcinogenic properties.[3] It is listed as an IARC Group 3 carcinogen.
Chemically, it is a chlorinated macrocyclic pentapeptide derived from the amino acids 3-phenyl-β-alanine, serine, dichloroproline, and aminobutyric acid.[4]
Although tributyltin (TBT) was recently found to induce apoptosis in different immune cells.[5]
References
- ↑ Zhou, ZH; Komiyama, M; Terao, K; Shimada, Y (1994). "Effects of cyclochlorotine on myofibrils in cardiomyocytes and on actin filament bundles in fibroblasts in vitro". Nat. Toxins 2 (6): 378–85. PMID 7704452.
- ↑ "Toxicology of Penicillium Islandicum". Nature 191 (4791): 864–865. 1961. Bibcode:1961Natur.191..864.. doi:10.1038/191864b0.
- ↑ Penicillium islandicum causes hepatic necrosis and has carcinogenic properties
- ↑ Kohei Mizutani, Yusuke Hirasawa, Yoshiko Sugita-Konishi, Naoki Mochizuki and Hiroshi Morita (2008). "Structural and Conformational Analysis of Hydroxycyclochlorotine and Cyclochlorotine, Chlorinated Cyclic Peptides from Penicillium islandicum". J. Nat. Prod. 71 (7): 1297–1300. doi:10.1021/np800150m. PMID 18558744.
- ↑ Lavastre, V; Girard, D (2002). "Tributyltin induces human neutrophil apoptosis and selective degradation of cytoskeletal proteins by caspases". J. Toxicol. Environ. Health Part A 65 (14): 1013–24. doi:10.1080/00984100290071270. PMID 12133234.