Cyclobutadiene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3-Cyclobutadiene | |||
| Other names
Cyclobutadiene [4]Annulene | |||
| Identifiers | |||
| 1120-53-2 | |||
| ChEBI | CHEBI:33657 | ||
| ChemSpider | 120626 | ||
| |||
| Jmol-3D images | Image Image | ||
| PubChem | 136879 | ||
| |||
| Properties | |||
| C4H4 | |||
| Molar mass | 52.07 g/mol | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Cyclobutadiene is the smallest [n]-annulene ([4]-annulene), an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula C4H4. It is believed to be in equilibrium between a pair of rectangular/nonplanar ground states and a square, excited triplet state based upon theoretical calculations,[1] and through spectroscopic[2] and crystallographic[3] investigation of substituted cyclobutadienes, in an argon matrix and inside carceplexes.[2] Though it has alternating single and double bonds, it is predicted triplet, unstable and antiaromatic by Hückel's rule,[4] because its ring has 4 π-electrons, and 4 is not twice an odd number. Some cyclobutadiene–metal compounds are stable, thought to be caused by the metal atom providing 2 more electrons to the system. Planar, rectangular distortion to a singlet ground state is caused by the Jahn–Teller effect.[5] Tetra-t-butyl substituted cyclobutadiene is sufficiently stable to gather x-ray crystallographic images of the structure which has a distorted, nonplanar configuration and double bond lengths longer than normal (1.464 vs expected 1.34)[3]
The pi electron energy of cyclobutadiene is higher than that of its open-chain counterpart, 1,3-butadiene, and it is therefore said to be anti-aromatic rather than aromatic. The electronic states of cyclobutadiene have been explored with a variety of computational methods.[6] The singlet ground state has a rectangular structure. The first excited state is a triplet with a square geometry. The rectangular structure is consistent with the existence of two different 1,2-dideuterio-1,3-cyclobutadiene stereoisomers. This indicates that the pi electrons are localized and therefore not considered to be aromatic. Cyclobutadiene is far from stable, it is highly reactive and has a very short lifetime. Cyclobutadiene dimerizes by a Diels-Alder reaction at 35 K. The monomeric form has been studied at higher temperatures by trapping with matrix isolation in a noble gas.
Synthesis
After numerous attempts, cyclobutadiene was first synthesized in 1965 by a team led by Rowland Pettit of the University of Texas, although they could not isolate it. Cyclobutadiene can be generated through degradation from a cyclobutadiene metal compound, for example cyclobutadieneiron tricarbonyl with ammonium cerium(IV) nitrate. This cyclobutadieneiron tricarbonyl complex was prepared from Fe2(CO)9 and cis-dichlorocyclobutene in a double dehydrohalogenation.[7][8]
Cyclobutadiene, when liberated from the iron complex, reacts with electron-deficient alkynes to form a Dewar benzene:[9]
The Dewar benzene converts to dimethyl phthalate on heating at 90°C.
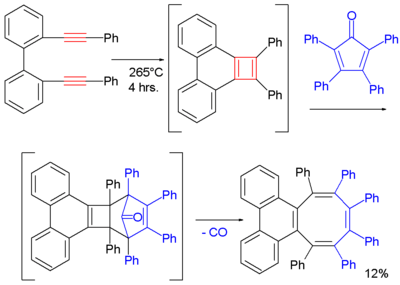
One cyclobutadiene derivative is also accessible through a [2+2]cycloaddition of a di-alkyne. In this particular reaction the trapping reagent is 2,3,4,5-tetraphenylcyclopenta-2,4-dienone and one of the final products (after expulsion of carbon monoxide) is a cyclooctatetraene:[10]
See also
- Cyclobutene
- Butadiene
References
- ↑ A theoretical study of the structure of cyclobutadiene H. Kollmar, V. Staemmler; J. Am. Chem. Soc., 1977, 99 (11), pp 3583–3587 doi:10.1021/ja00453a009
- ↑ 2.0 2.1 The Taming of Cyclobutadiene Donald J. Cram, Martin E. Tanner, Robert Thomas; Angewandte Chemie International Edition in English Volume 30, Issue 8, pages 1024–1027, August 1991
- ↑ 3.0 3.1 Structure of Tetra-tert-butylcyclobutadiene Hermann Irngartinger, Norbert Riegler1. Klaus-Dieter Malsch, Klaus-Albert Schneider, Günther Maier; Angewandte Chemie International Edition in English Volume 19, Issue 3, pages 211–212, March 1980 doi:10.1002/anie.198002111
- ↑ Huckel Theory II J. M. LoBue, Copyright 2002 by the Division of Chemical Education, Inc., American Chemical Society. Link
- ↑ A simple quantum mechanical model that illustrates the Jahn-Teller effect Peter Senn; J. Chem. Educ., 1992, 69 (10), p 819 doi:10.1021/ed069p819
- ↑ Balkova, A.; Bartlett, R. J. J. Chem. Phys. 1994, 101, 8972– 8987
- ↑ Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes G. F. Emerson, L. Watts, R. Pettit; J. Am. Chem. Soc.; 1965; 87(1); 131-133. First Page
- ↑ Iron, tricarbonyl (η4-1,3-cyclobutadiene)- R. Pettit and J. Henery Organic Syntheses, Coll. Vol. 6, p.310 (1988); Vol. 50, p.21 (1970) Link
- ↑ Cyclobutadiene L. Watts, J. D. Fitzpatrick, R. Pettit J. Am. Chem. Soc.; 1965; 87(14); 3253-3254. doi:10.1021/ja01092a049
- ↑ Revisit of the Dessy-White Intramolecular Acetylene-Acetylene [2 + 2] Cycloadditions Chung-Chieh Lee, Man-kit Leung, Gene-Hsiang Lee, Yi-Hung Liu, and Shie-Ming Peng J. Org. Chem.; 2006; 71(22) pp 8417 - 8423; (Article) doi:10.1021/jo061334v
| ||||||||||||||
| ||||||||||||||