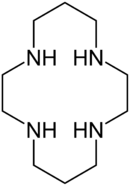
Cyclam
 | |
| Names | |
|---|---|
| IUPAC name
1,4,8,11-Tetraazacyclotetradecane | |
| Identifiers | |
| 295-37-4 | |
| ChEBI | CHEBI:37401 |
| ChEMBL | ChEMBL125150 |
| ChemSpider | 58489 |
| |
| Jmol-3D images | Image |
| PubChem | 64964 |
| |
| Properties | |
| Molecular formula |
C10H24N4 |
| Molar mass | 200.32 g·mol−1 |
| Melting point | 185 °C (365 °F; 458 K) |
| 5 g/100 mL (20 °C) | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. It is a white solid that is soluble in water. The compound is notable as a macrocyclic ligand, which binds strongly to many transition metal cations.[1] The compound was first prepared by the reaction of 1,3-dibromopropane and ethylenediamine.[2]
The compound features four secondary amines. Its complexes therefore can exist as several diastereomers, depending on the relative orientation of the N-H centres. Its complexes feature alternating five- and six-membered chelate rings. The closely related ligand cyclen ((CH2CH2NH)4) forms only five-membered C2N2M chelate rings and tends not to form square-planar complexes.
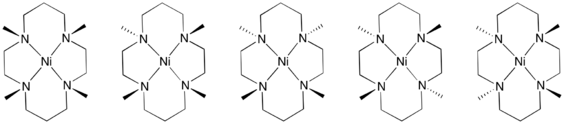
N-Alkyl derivatives
Metal-cyclam complexes are prone to oxidative degradation, which is initiated by deprotonation of the secondary amine. This flaw led to the development of cyclam derivatives wherein the NH centres are replaced by tertiary amines. For example the tetramethyl derivatives are readily prepared by methylation using formaldehyde and formic acid.[1] These oxidatively robust derivatives of cyclam have enabled a number of metal-dioxygen complexes.[3]
References
- ↑ 1.0 1.1 E. Kent Barefield "Coordination chemistry of N-tetraalkylated cyclam ligands—A status report" Coordination Chemistry Reviews 2010, volume 254, pages 1607–1627.doi:10.1016/j.ccr.2010.03.007
- ↑ J. van Alphen "On Aliphatic Polyamines IV" Rec. Trav. Chim. Pays-Bas 1937, volume 56, 343–350.
- ↑ J. Cho, R. Sarangi and W. Nam, "Mononuclear Metal-O2 Complexes Bearing Macrocyclic N-Tetramethylated Cyclam Ligands", Accounts of Chemical Research 2012, 45, 1321-1330. doi:10.1021/ar3000019