Critical radius
Critical radius is the minimum size that must be formed by atoms or molecules clustering together (in a gas, liquid or solid matrix) before a new-phase inclusion (a bubble, a droplet, or a solid particle) is stable and begins to grow. Formation of such stable "nuclei" is called nucleation.
In precipitation models this is generally a prelude to models of the growth process itself. Sometimes precipitation is rate-limited by the nucleation process. This happens for example before one takes a cup of superheated water from a microwave and, when jiggling it against dust particles on the wall of the cup, enables "heterogeneous" nucleation that then rapidly converts much of that water into steam.
If the change in phase forms a crystalline solid in a liquid matrix, the atoms might then form a dendrite. The crystal growth continues in three dimensions, the atoms attaching themselves in certain preferred directions, usually along the axes of a crystal, forming a characteristic tree-like structure of a dendrite.
Example: the critical radius for spheric-like dendride in an ideal system can be determined from its Gibbs free energy
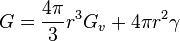
where 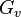 is the Gibbs volume energy and
is the Gibbs volume energy and 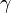 is the interfacial energy. The critical radius
is the interfacial energy. The critical radius 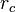 is found by setting the derivative of
is found by setting the derivative of 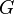 equal to zero
equal to zero
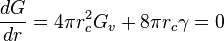
yielding
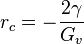 ,
,
where 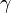 is the surface energy, and
is the surface energy, and 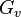 is Gibbs energy per volume.
is Gibbs energy per volume.
See also
- Nucleation
- Homogeneous nucleation
- Heterogeneous nucleation
- Ostwald ripening
References
- N.H.Fletcher, Size Effect in Heterogeneous Nucleation, J.Chem.Phys.29, 1958, 572.