Coxsackie virus and adenovirus receptor
Coxsackievirus and adenovirus receptor (CAR) is a protein that in humans is encoded by the CXADR gene.[1][2][3] The protein encoded by this gene is a type I membrane receptor for group B coxsackie viruses and subgroup C adenoviruses. The human CAR gene (CXADR) is found on chromosome 21. Alternative splicing is known to produce at least 2 splice variants known as hCAR1 and hCAR2 and are each composed of at least 7 exons. Pseudogenes of this gene are found on chromosomes 15, 18, and 21.[3]
Structure
CAR is a transmembrane bound protein with two Ig-like extracellular domains, a transmembrane domain, and a cytoplasmic domain. The N-terminal segment comprises the two extracellular domains (D1 and D2). D1 is most distal from the membrane and contains a V/Ig-like fold whereas D2 is more proximal and
Expression
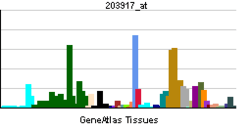
The protein is found to be expressed in various regions of the body including the heart, brain, and, more generally, epithelial and endothial cells. Moreover, CAR expression is not found in normal or tumour cell lines. Expression of CAR in endothial cells can be regulated by treatment with drugs. [4] [5]
Function
CAR is strongly expressed in the developing central nervous system where it is thought to mediate neurite outgrowth. In contrast,expression of CAR is undetectable in the adult nervous system. It functions as a homophilic and heterophilic cell adhesion molecule through its interactions with extracellular matrix glycoproteins such as: fibronectin, agrin, laminin-1 and tenascin-R.[6] In addition, it is thought to regulate the cytoskeleton through interactions with actin and microtubules. Moreover, its cytoplasmic domain contains putative phosphorylation sites and a PDZ-interaction motif which suggests a scaffolding role.
References
- ↑ Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW (Mar 1997). "Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5". Science 275 (5304): 1320–3. doi:10.1126/science.275.5304.1320. PMID 9036860.
- ↑ Tomko RP, Xu R, Philipson L (May 1997). "HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses". Proc Natl Acad Sci U S A 94 (7): 3352–6. doi:10.1073/pnas.94.7.3352. PMC 20373. PMID 9096397.
- ↑ 3.0 3.1 "Entrez Gene: CXADR coxsackie virus and adenovirus receptor".
- ↑ Funke C, Farr M, Werner B, Dittmann S, Uberla K, Piper C, Niehaus K, Horstkotte D (Apr 2010). "Antiviral effect of Bosentan and Valsartan during coxsackievirus B3 infection of human endothelial cells.". Journal of General Virology 91 (8): 1959–1570. doi:10.1099/vir.0.020065-0. PMID 20392896.
- ↑ Werner B, Dittmann S, Funke C, Überla K, Piper C, Niehaus K, Horstkotte D, Farr M. (Dec 2013). "Effect of lovastatin on coxsackievirus B3 infection in human endothelial cells.". Inflammation Research 63 (4): 267–276. doi:10.1007/s00011-013-0695-z. PMID 24316867.
- ↑ Patzke C, Max KE, Behlke J, Schreiber J, Schmidt H, Dorner AA, Kröger S, Henning M, Otto A, Heinemann U, Rathjen FG (Feb 2010). "The Coxsackievirus-Adenovirus Receptor reveals complex homophilic and heterophilic interactions on neural cells". Journal of Neuroscience 30 (8): 2897–2910. doi:10.1523/JNEUROSCI.5725-09.2010. PMID 20181587.
Further reading
- Carson SD (2002). "Receptor for the group B coxsackieviruses and adenoviruses: CAR.". Rev. Med. Virol. 11 (4): 219–26. doi:10.1002/rmv.318. PMID 11479928.
- Selinka HC, Wolde A, Sauter M et al. (2004). "Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism.". Med. Microbiol. Immunol. 193 (2–3): 127–31. doi:10.1007/s00430-003-0193-y. PMID 12920584.
- Carson SD, Chapman NN, Tracy SM (1997). "Purification of the putative coxsackievirus B receptor from HeLa cells". Biochem. Biophys. Res. Commun. 233 (2): 325–8. doi:10.1006/bbrc.1997.6449. PMID 9144533.
- Bergelson JM, Krithivas A, Celi L et al. (1998). "The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses". J. Virol. 72 (1): 415–9. PMC 109389. PMID 9420240.
- Fechner H, Haack A, Wang H et al. (2000). "Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers". Gene Ther. 6 (9): 1520–35. doi:10.1038/sj.gt.3301030. PMID 10490761.
- Bowles KR, Gibson J, Wu J et al. (1999). "Genomic organization and chromosomal localization of the human Coxsackievirus B-adenovirus receptor gene". Hum. Genet. 105 (4): 354–9. doi:10.1007/s004390051114. PMID 10543405.
- Bewley MC, Springer K, Zhang YB et al. (1999). "Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR". Science 286 (5444): 1579–83. doi:10.1126/science.286.5444.1579. PMID 10567268.
- Tomko RP, Johansson CB, Totrov M et al. (2000). "Expression of the adenovirus receptor and its interaction with the fiber knob". Exp. Cell Res. 255 (1): 47–55. doi:10.1006/excr.1999.4761. PMID 10666333.
- van Raaij MJ, Chouin E, van der Zandt H et al. (2001). "Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1.7 A resolution". Structure 8 (11): 1147–55. doi:10.1016/S0969-2126(00)00528-1. PMID 11080637.
- Cohen CJ, Gaetz J, Ohman T, Bergelson JM (2001). "Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting". J. Biol. Chem. 276 (27): 25392–8. doi:10.1074/jbc.M009531200. PMID 11316797.
- Noutsias M, Fechner H, de Jonge H et al. (2001). "Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections". Circulation 104 (3): 275–80. doi:10.1161/01.cir.104.3.275. PMID 11457744.
- Thoelen I, Magnusson C, Tågerud S et al. (2001). "Identification of alternative splice products encoded by the human coxsackie-adenovirus receptor gene". Biochem. Biophys. Res. Commun. 287 (1): 216–22. doi:10.1006/bbrc.2001.5535. PMID 11549277.
- He Y, Chipman PR, Howitt J et al. (2001). "Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor". Nat. Struct. Biol. 8 (10): 874–8. doi:10.1038/nsb1001-874. PMID 11573093.
- Cohen CJ, Shieh JT, Pickles RJ et al. (2002). "The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction". Proc. Natl. Acad. Sci. U.S.A. 98 (26): 15191–6. doi:10.1073/pnas.261452898. PMC 65005. PMID 11734628.
- Law LK, Davidson BL (2002). "Adenovirus serotype 30 fiber does not mediate transduction via the coxsackie-adenovirus receptor". J. Virol. 76 (2): 656–61. doi:10.1128/JVI.76.2.656-661.2002. PMC 136819. PMID 11752156.
- van't Hof W, Crystal RG (2002). "Fatty acid modification of the coxsackievirus and adenovirus receptor". J. Virol. 76 (12): 6382–6. doi:10.1128/JVI.76.12.6382-6386.2002. PMC 136239. PMID 12021372.
- Walters RW, Freimuth P, Moninger TO et al. (2002). "Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape". Cell 110 (6): 789–99. doi:10.1016/S0092-8674(02)00912-1. PMID 12297051.
| |||||||||||||||||||||||||