Cortistatins
 | |
| Names | |
|---|---|
| IUPAC name
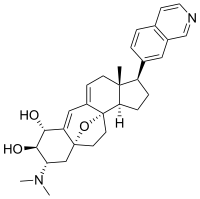
(1R,2R,3S,5R,8β,17β)-3-(Dimethylamino)-17-(isoquinolin-7-yl)-5,8-epoxy-9,19-cyclo-9,10-secoandrosta-9(11),10-diene-1,2-diol | |
| Other names
Cortistatine A | |
| Identifiers | |
| 882976-95-6 | |
| ChEBI | CHEBI:67171 |
| ChemSpider | 9736681 |
| |
| Jmol-3D images | Image |
| PubChem | 11561907 |
| |
| Properties | |
| Molecular formula |
C30H36N2O3 |
| Molar mass | 472.62 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
The cortistatins are a group of steroidal alkaloids first isolated in 2006 from the marine sponge Corticium simplex.[1] The cortistatins inhibit proliferation of human umbilical vein endothelial cells (HUVECs) with high selectivity, with cortistatin A being the most potent compound in the class.[2] Cortistatin A represses viral replication in cells infected with HIV via binding to the Tat protein. [3]
The unique chemical structure and potent biological activity of these compounds have stimulated interest in their total synthesis and further biological evaluation.[4][5]
Chemical structures
-

Cortistatin A
-
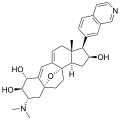
Cortistatin B
-
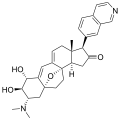
Cortistatin C
-
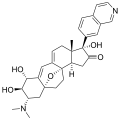
Cortistatin D
-
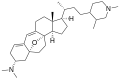
Cortistatin E
-
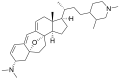
Cortistatin F
-
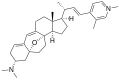
Cortistatin G
-
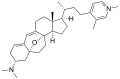
Cortistatin H
-

Cortistatin J
-
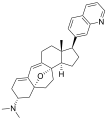
Cortistatin K
-
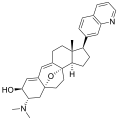
Cortistatin L
References
- ↑ Aoki, S; Watanabe, Y; Sanagawa, M; Setiawan, A; Kotoku, N; Kobayashi, M (2006). "Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex". Journal of the American Chemical Society 128 (10): 3148–9. doi:10.1021/ja057404h. PMID 16522087.
- ↑ Aoki, S; Watanabe, Y; Tanabe, D; Arai, M; Suna, H; Miyamoto, K; Tsujibo, H; Tsujikawa, K; Yamamoto, H (2007). "Structure-activity relationship and biological property of cortistatins, anti-angiogenic spongean steroidal alkaloids". Bioorganic & Medicinal Chemistry 15 (21): 6758–62. doi:10.1016/j.bmc.2007.08.017. PMID 17765550.
- ↑ Mousseau, G.; Clementz, M. A.; Bakeman, W. N.; Nagarsheth, N.; Cameron, M.; Shi, J.; Baran, P.; Fromentin, R. M.; Chomont, N.; Valente, S. T. (2012). "An Analog of the Natural Steroidal Alkaloid Cortistatin a Potently Suppresses Tat-Dependent HIV Transcription". Cell Host & Microbe 12 (1): 97–108. doi:10.1016/j.chom.2012.05.016. PMC 3403716. PMID 22817991.
- ↑ Chen, David Yu-Kai; Tseng, Chih-Chung (2010). "Chemistry of the cortistatins–a novel class of anti-angiogenic agents". Organic & Biomolecular Chemistry 8 (13): 2900. doi:10.1039/C003935G.
- ↑ Hardin Narayan, Alison R.; Simmons, Eric M.; Sarpong, Richmond (2010). "Synthetic Strategies Directed Towards the Cortistatin Family of Natural Products". European Journal of Organic Chemistry 2010 (19): 3553. doi:10.1002/ejoc.201000247.