Coprine
 | |
| Names | |
|---|---|
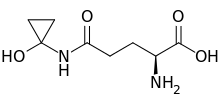
| IUPAC name
N-(1-Hydroxycyclopropyl)-L-glutamine | |
| Other names
(S)-2-Amino-5-[(1-hydroxycyclopropyl)amino]-5-oxopentanoate | |
| Identifiers | |
| 58919-61-2 | |
| ChemSpider | 97180 |
| |
| Jmol-3D images | Image |
| PubChem | 108079 |
| |
| Properties | |
| Molecular formula |
C8H14N2O4 |
| Molar mass | 202.21 g·mol−1 |
| Melting point | 197 °C (387 °F; 470 K) [1] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Coprine is a mycotoxin. It was first isolated from common inkcap (Coprinopsis atramentaria). It also occurs in other mushrooms in the genus Coprinus[2] and in brawny bolete (Boletus torsus).[3]
When combined with the consumption of alcohol, it causes "Coprinus syndrome".[4]:284[5] Symptoms include facial reddening, nausea, vomiting, malaise, agitation, palpitations and tingling in limbs, and arise five to ten minutes after consumption of alcohol.[4]:288 The signs are similar to those induced by disulfiram (Antabuse) and subsequent alcohol consumption. If no more alcohol is consumed, the symptoms will generally subside over two or three hours. Symptom severity is proportional to the amount of alcohol consumed.
Coprine is metabolized into glutamic acid and 1-aminocyclopropanol, the biologically active substance which inhibits the enzyme acetaldehyde dehydrogenase. This enzyme is involved in the metabolism of alcohol. When it is blocked, acetaldehyde accumulates causing the adverse reaction.
References
- ↑ RÖMPP Online – Version 3.4, Stuttgart: Thieme Chemistry, 2009
- ↑ "Disulfiramlike Mushroom Toxicity". Medscape.
- ↑ Kiwitt U, Laatsch H. (1994). "Coprin in Boletus torosus: Beruht die angebliche Alkoholunverträglichkeit durch den Verzehr des Netzstieligen Hexenröhrlings (Boletus luridus) auf einer Verwechslung?" [Coprine in Boletus torosus: Is the alleged alcohol hypersensitivity by ingestion of B. luridus caused by a mistake?] (PDF). Zeitschrift für Mykologie (in German) 60 (2): 423–30.
- ↑ 4.0 4.1 Benjamin, Denis R. (1995). Mushrooms: poisons and panaceas—a handbook for naturalists, mycologists and physicians. New York: WH Freeman and Company. ISBN 0-7167-2600-9.
- ↑ Michelot, D. (1992). "Poisoning by Coprinus atramentarius". Natural toxins 1 (2): 73–80. doi:10.1002/nt.2620010203. PMID 1344910.