Collision frequency
Main article: Collision theory
Collision frequency is defined in chemical kinetics, in the background of theoretical kinetics, as the average number of collisions between reacting molecules per unit of time per moles of reactant. Its symbol is Z. For a gas-phase bimolecular reaction, Z is
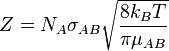
- NA is Avogadro's number
- σAB is the reaction cross section
- kB is Boltzmann constant
- T is temperature in kelvin
- μAB is the reduced mass of the reactants