Coccolithophore
| Coccolithaceae
Temporal Range: Late Triassic - Present[1] | |
|---|---|
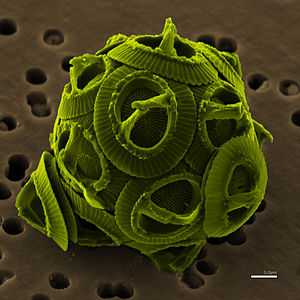 | |
| The coccolithophore Gephyrocapsa oceanica | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Chromalveolata |
| Phylum: | Haptophyta |
| Class: | Coccolithophyceae Rothmaler, 1951 |
| Order: | Isochrysidales, Coccolithales |
| Synonyms | |
| |
A coccolithophore (the adjective coccolithophorid is often incorrectly used as if it were a noun[2]) is a unicellular, eukaryotic phytoplankton (alga). They belong either to the kingdom Protista, according to Robert Whittaker's Five kingdom classification, or Chromalveolata, according to the newer Thomas Cavalier-Smith Biological Classification system. Within the Chromalveolata, the coccolithophorids are in the phylum or division Haptophyta, class Coccolithophyceae.[3] Coccolithophorids are distinguished by special calcium carbonate plates (or scales) of uncertain function called coccoliths, which are also important microfossils. However, there are Coccolithophyceae species lacking coccoliths (e.g. in genus Prymnesium), so not every member of Coccolithophyceae (Prymnesiophyceae) is coccolithophorid.[4] Coccolithophores are almost exclusively marine and are found in large numbers throughout the sunlight zone of the ocean.
The most abundant species of coccolithophore, Emiliana huxleyi, belongs to the order Isochrysidales and family Noëlaerhabdaceae.[3] It is found in temperate, subtropical, and tropical oceans.[5] This makes E. huxleyi an important part of the planktonic base of a large proportion of marine food webs. It is also the fastest growing coccolithophore in laboratory cultures.[6] It is studied for the extensive blooms it forms in nutrient depleted waters after the reformation of the summer thermocline.[7] and for its production of a group resistant alkenones commonly used by earth scientists as a means to estimate past sea surface temperatures.[8] Coccolithophores are of particular interest to those studying global climate change because as ocean acidity increases, their coccoliths may become even more important as a carbon sink.[9] Furthermore, management strategies are being employed to prevent eutrophication-related coccolithophore blooms, as these blooms lead to a decrease in nutrient flow to lower levels of the ocean.[10]
-

Coccolithus pelagicus.
-

The milky blue colour of this phytoplankton bloom in Barents Sea strongly suggests that it contains coccolithophores.
-

Phaeocystis bloom in Nord-Pas-de-Calais.
Structure
Coccolithophores are spherical cells about 5–100 micrometres across, enclosed by calcareous plates called coccoliths, which are about 2–25 micrometres across. Each cell contains two brown chloroplasts which surround the nucleus.[11]
Exoskeleton (coccosphere)
Each unicellular plankton is enclosed in its own collection of coccoliths, the calcified scales, which make up its exoskeleton or coccosphere.[12] The coccoliths are created inside the cell and while some species maintain a single layer throughout life only producing new coccoliths as the cell grows, others continually produce and shed coccoliths.
Composition
The primary constituent of coccoliths is calcium carbonate, or chalk. Calcium carbonate is transparent, so the organisms’ photosynthetic activity is not compromised by encapsulation in a coccosphere.[13]
Formation
Coccoliths are produced by a biomineralization process known as coccolithogenesis.[11] Generally, calcification of coccoliths occurs in the presence of light, and these scales are produced much more during the exponential phase of growth than the stationary phase.[14] Although not yet entirely understood, the biomineralization process is tightly regulated by calcium signaling. Calcite formation begins in the golgi complex where protein templates nucleate the formation of CaCO3 crystals and complex acidic polysaccharides control the shape and growth of these crystals.[15] As each scale is produced, it is exported in a Golgi-derived vesicle and added to the inner surface of the coccosphere. This means that the most recently produced coccoliths may lie beneath older coccoliths.[16] Depending upon the phytoplankton’s stage in the life cycle, two different types of coccoliths may be formed. Holococcoliths are produced only in the haploid phase, lack radial symmetry, and are composed of anywhere from hundreds to thousands of similar minute (ca 0.1 µm) rhombic calcite crystals. These crystals are thought to form at least partially outside the cell. Heterococcoliths occur only in the diploid phase, have radial symmetry, and are composed of relatively few complex crystal units (<100) . Although they are rare, combination coccospheres, which contain both holococcoliths and heterococcoliths, have been observed in the plankton recording coccolithophore life cycle transitions. Finally, the coccospheres of some species are highly modified with various appendages made of specialized coccoliths.[17]
Function
While the exact function of the coccosphere is unclear, many potential functions have been proposed. Most obviously coccoliths may protect the phytoplankton from predators. In addition, these exoskeletons may confer an advantage in energy production, as coccolithogenesis seems highly coupled with photosynthesis. Organic precipitation of calcium carbonate from bicarbonate solution produces free carbon dioxide directly within the cellular body of the alga, this additional source of gas is then available to the Coccolithophore for photosynthesis. It has been suggested that they may provide a cell-wall like barrier to isolate intracellular chemistry from the marine environment.[18] More specific, defensive properties of coccoliths may include protection from osmotic changes, chemical or mechanical shock, and short-wavelength light.[19] It has also been proposed that the added weight of multiple layers of coccoliths allows the organism to sink to lower, more nutrient rich layers of the water and conversely, that coccoliths add buoyancy, stopping the cell from sinking to dangerous depths.[20] Coccolith appendages have also been proposed to serve several functions, such as inhibiting grazing by zooplankton.[17]
Uses
Coccoliths are the main component of the Chalk, a Late Cretaceous rock formation which outcrops widely in southern England and forms the White Cliffs of Dover, and of other similar rocks in many other parts of the world. At the present day sedimented coccoliths are a major component of the calcareous oozes that cover up to 35% of the ocean floor and is kilometres thick in places.[15] Because of their abundance and wide geographic ranges, the coccoliths which make up the layers of this ooze and the chalky sediment formed as it is compacted serve as valuable microfossils.
Cellular anatomy
Enclosed in each coccosphere is a single cell with membrane bound organelles. Two large chloroplasts with brown pigment are located on either side of the cell and surround the nucleus, mitochondria, golgi apparatus, endoplasmic reticulum, and other organelles. Each cell also has two flagellar structures, which are involved not only in motility, but also in mitosis and formation of the cytoskeleton.[21] In some species, a functional or vestigial haptonema is also present.[19] This structure, which is unique to haptophytes, coils and uncoils in response to environmental stimuli. Although poorly understood, it has been proposed to be involved in prey capture.[21]
Ecology
Life history strategy
The life cycle of coccolithophores is characterized by an alternation of diploid and haploid phases. They alternate from the haploid to diploid phase through syngamy and from diploid to haploid through meiosis. In contrast with most organisms with alternating life cycles, asexual reproduction by mitosis is possible in both phases of the life cycle.[16] Both abiotic and biotic factors may affect the frequency with which each phase occurs.[22]
Coccolithophores reproduce asexually through binary fission. In this process the coccoliths from the parent cell are divided between the two daughter cells. There have been suggestions stating the possible presence of a sexual reproduction process due to the diploid stages of the coccolithophores, but this process has never been observed.[23] K or r- selected strategies of coccolithophores depend on their life cycle stage. When coccolithophores are diploid, they are r-selected. In this phase they tolerate a wider range of nutrient compositions. When they are haploid they are K- selected and are often more competitive in stable low nutrient environments.[23] Most coccolithophores are K strategist and are usually found on nutrient-poor surface waters. They are poor competitors when compared to other phytoplankton and thrive in habitats where other phytoplankton would not survive.[13] These two stages in the life cycle of coccolithophores occur seasonally, where more nutrition is available in warmer seasons and less is available in cooler seasons. This type of life cycle is known as a complex heteromorphic life cycle.[23]
Global distribution

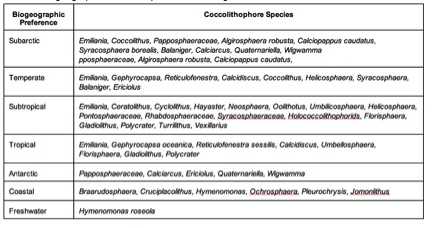
Coccolithophores occur throughout the world ocean. Their distribution varies vertically by stratified layers in the ocean and geographically by different temporal zones.[24] While most modern coccolithophores can be located in their associated stratified oligotrophic conditions, the most abundant areas of coccolithophores where there is the highest species diversity are located in subtropical zones with a temperate climate.[25] While water temperature and the amount of light intensity entering the water’s surface are the more influential factors in determining where species are located, the ocean currents also can determine the location where certain species of coccolithophores are found.[26]
Although motility and colony formation vary according to the life cycle of different coccolithophore species, there is often alternation between a motile, haploid phase, and a non-motile diploid phase. In both phases, the organism’s dispersal is largely due to ocean currents and circulation patterns.[15]
Within the Pacific Ocean, approximately 90 species have been identified with six separate zones relating to different Pacific currents that contain unique groupings of different species of coccolithophores.[27] The highest diversity of coccolithophores in the Pacific Ocean was in an area of the ocean considered the Central North Zone which is an area between 30 oN and 5 oN, composed of the North Equatorial Current and the Equatorial Countercurrent. These two currents move in opposite directions, east and west, allowing for a strong mixing of waters and allowing a large variety of species to populate the area.[27]
In the Atlantic Ocean, the most abundant species are E. huxleyi and Florisphaera profunda with smaller concentrations of the species Umbellosphaera irregularis, Umbellosphaera tenuis and different species of Gephyrocapsa.[27] Deep-dwelling coccolithophore species abundance is greatly affected by nutricline and thermocline depths. These coccolithophores increase in abundance when the nutricline and thermocline are deep and decrease when they are shallow.[28]
The complete distribution of coccolithophores is currently not known and some regions, such as the Indian Ocean, are not as well known as other locations in the Pacific and Atlantic Oceans. It is also very hard to explain distributions due to multiple constantly changing factors involving the ocean properties, such as coastal and equatorial upwelling, frontal systems, benthic environments, unique oceanic topography, and pockets of isolated high or low water temperatures.[17]
Table 1 and Table 2 show simplified specific species preferences based on geographic location and water depth respectively. These do not contain all coccolithophore species and are just a simplification of major, more well-known species.
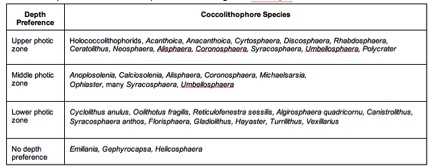
The upper photic zone is low in nutrient concentration, high in light intensity and penetration, and usually higher in temperature. The lower photic zone is high in nutrient concentration, low in light intensity and penetration and relatively cool. The middle photic zone is an area that contains the same values in between that of the lower and upper photic zones.[25]
Effect of global climate change on distribution and abundance
Recent studies show that climate change has direct and indirect impacts on Coccolithophore distribution and productivity. They will inevitably be affected by the increasing temperatures and thermal stratification of the top layer of the ocean, since these are prime controls on their ecology, although it is not clear whether global would result in net increase or decrease of coccolithophores. It has been suggested that since they calcify ocean acidification due to increasing carbon dioxide could severely affect coccolithophores.[28]
Role in the food web
Coccolithophores are one of the more abundant primary producers in the ocean. As such, they are a large contributor to the primary productivity of the tropical and subtropical oceans, however, exactly how much has yet to have been recorded.[29]
Dependence on nutrients
The ratio between the concentrations of nitrogen, phosphorus and silicate in particular areas of the ocean dictates competitive dominance within phytoplankton communities. Each ratio essentially tips the odds in favor of either diatoms or more other groups of phytoplankton, such as coccolithophores. A low silicate to nitrogen and phosphorus ratio allows coccolithophores to outcompete other phytoplankton species; however, when silicate to phosphorus to nitrogen ratios are high coccolithophores are outcompeted by diatoms. The increase in agricultural processes lead to eutrophication of waters and thus, coccolithophore blooms in these high nitrogen and phosphorus, low silicate environments.[10]
Impact on water column productivity
The calcite in calcium carbonate allows coccoliths to scatter more light than they absorb. This has two important consequences: 1) Surface waters become brighter, meaning they have a higher albedo, and 2) there is induced photoinhibition, meaning deeper waters become darker. Hence, a high concentration of coccoliths leads to a simultaneous increase in surface water temperature and decrease in the temperature of deeper waters. This results in more stratification in the water column and a decrease in the vertical mixing of nutrients. However, a recent study estimated that the overall effect of coccolithophores on the increased in radiative forcing of the ocean is less than that from anthropogenic factors.[30] Therefore, the overall result of large blooms of coccolithophores is a decrease in water column productivity, rather than a contribution to global warming.
Predator-prey interactions
Their predators include the common predators of all phytoplankton including small fish, zooplankton, and shellfish larvae.[13][31] Viruses specific to this species have been isolated from several locations worldwide and appear to play a major role in spring bloom dynamics.
Toxicity
No environmental evidence of coccolithophore toxicity has been reported, but they belong to the class Prymnesiophyceae which contain orders with toxic species. Toxic species have been found in the genera Prymnesium Massart and Chrysochromulina Lackey. Members of the genus Prymnesium have been found to produce haemolytic compounds, the agent responsible for toxicity. Some of these toxic species are responsible for large fish kills and can be accumulated in organisms such as shellfish; transferring it through the food chain. In laboratory tests for toxicity members of the oceanic coccolithophore genera Emiliania, Gephyrocapsa, Calcidiscus and Coccolithus were shown to be non-toxic as were species of the coastal genus Hymenomonas, however several species of Pleurochrysis and Jomonlithus, both coastal genera were toxic to Artemia.[31]
Community interactions
Coccolithophorids are predominantly found as single, free-floating haploid or diploid cells.[24]
Competition
Most phytoplankton need sunlight and nutrients from the ocean to survive, so they thrive in areas with large inputs of nutrient rich water upwelling from the lower levels of the ocean. Most coccolithophores, only require sunlight for energy production and have a higher ratio of nitrate uptake over ammonium uptake (nitrogen is required for growth and can be used directly from nitrate but not ammonium). Because of this they thrive in still, nutrient-poor environments where other phytoplankton are starving.[32] Trade-offs associated with these faster growth rates, however, include a smaller cell radius and lower cell volume than other types of phytoplankton.
Viral infection and coevolution
Giant DNA-containing viruses are known to lytically infect coccolithophores, particularly E. huxleyi. These viruses, known as E. huxleyi viruses (EhVs), appear to infect the coccosphere coated diploid phase of the life cycle almost exclusively. It has been proposed that as the haploid organism is not infected and therefore not affected by the virus, the co-evolutionary “arms race” between coccolithophores and these viruses does not follow the classic Red Queen evolutionary framework, but instead a “Cheshire Cat” ecological dynamic.[33] More recent work has suggested that viral synthesis of sphingolipids and induction of programmed cell death provides a more direct link to study a Red Queen-like coevolutionary arms race at least between the coccolithoviruses and diploid organism.[22]
Importance in global climate change
Impact on the carbon cycle
Coccolithophores have both long and short term effects on the carbon cycle. The production of coccoliths requires the uptake of dissolved inorganic carbon and calcium. Calcium carbonate and carbon dioxide are produced from calcium and bicarbonate by the following chemical reaction:
- Ca2+ + 2HCO3− ←→ CaCO3 + CO2 + H2O.[34]
Because coccolithophores are photosynthetic organisms, they are able to use some of the CO2 released in the calcification reaction for photosynthesis.[35]
However, the production of calcium carbonate drives surface alkalinity down, and in conditions of low alkalinity the CO2 is instead released back into the atmosphere.[36] As a result of this, researchers have postulated that large blooms of coccolithophores may contribute to global warming in the short term.[37] A more widely accepted idea, however, is that over the long term coccolithophores contribute to an overall decrease in atmospheric CO2 concentrations. During calcification two carbon atoms are taken up and one of them becomes trapped as calcium carbonate. This calcium carbonate sinks to the bottom of the ocean in the form of coccoliths and becomes part of sediment; thus, coccolithophores provide a sink for emitted carbon, mediating the effects of greenhouse gas emissions.[37]
Evolutionary responses to ocean acidification
Research also suggests that ocean acidification due to increasing concentrations of CO2 in the atmosphere may affect the calcification machinery of coccolithophores. This may not only affect immediate events such as increases in population or coccolith production, but also may induce evolutionary adaptation of coccolithophore species over longer periods of time. For example, coccolithophores use H+ ion channels in to constantly pump H+ ions out of the cell during coccolith production. This allows them to avoid acidosis, as coccolith production would otherwise produce a toxic excess of H+ ions. When the function of these ion channels is disrupted, the coccolithophores stop the calcification process to avoid acidosis, thus forming a feedback loop.[38] Low ocean alkalinity, impairs ion channel function and therefore places evolutionary selective pressure on coccolithophores and makes them (and other ocean calcifiers) vulnerable to ocean acidification.[39] In 2008, field evidence indicating an increase in in calcification of newly formed ocean sediments containing coccolithophores bolstered the first ever experimental data showing that an increase in ocean CO2 concentration results in an increase in calcification of these organisms. Decreasing coccolith mass is related to both the increasing concentrations of CO2 and decreasing concentrations of CO3 in the world’s oceans. This lower calcification is assumed to put coccolithophores at ecological disadvantage. Some species like Calcidiscus leptoporus, however, are not affected in this way, while the most abundant coccolithophore species, E. huxleyi is.[38] Also, highly calcified coccolithophorids have been found in conditions of low CO−3 contrary to predictions.[9] Understanding the effects of increasing ocean acidification on coccolithophore species is absolutely essential to predicting the future chemical composition of the ocean, particularly its carbonate chemistry. Viable conservation and management measures will come from future research in this area. Groups like the European-based CALMARO[40] are monitoring the responses of coccolithophore populations to varying pH’s and working to determine environmentally sound measures of control.
Impact on nannofossil record
Coccolith fossils are prominent and valuable calcareous nannofossils (see Micropaleontology). Of particular interest are fossils dating back to the Palaeocene-Eocene Thermal Maximum 55 million years ago. This period is thought to correspond most directly to the current levels of CO2 in the ocean.[41] Finally, field evidence of coccolithophore fossils in rock were used to show that the deep-sea fossil record bears a rock record bias similar to the one that is widely accepted to affect the land-based fossil record.[42]
Impact on the oceans
The coccolithophorids help in regulating the temperature of the oceans. They thrive in warm seas and release DMS (demethyl sulphide) into the air whose nuclei help to produce thicker clouds to block the sun. When the oceans cool, the number of coccolithophorids decrease and the amount of clouds also decrease. When there are fewer clouds blocking the sun, the temperature also rises. This, therefore, maintains the balance and equilibrium of nature.
Modern Culture
The coccolithophores are featured on the new design for the 500 Kroner bank note.[43]
See also
- CLAW hypothesis
- Dimethyl sulfide
- Dimethylsulfoniopropionate
- Emiliania huxleyi virus 86
- Pleurochrysis carterae
References
- ↑ Bown, P.R.; Lees, J.A.; Young, J.R. (August 17, 2004). "Calcareous nannoplankton evolution and diversity through time". In Thierstein, Hans R.; Young, Jeremy R. Coccolithophores-from molecular processes to global impact. Berlin: Springer. pp. 481–508. ISBN 9783540219286..
- ↑ International Nannoplankton Association
- ↑ 3.0 3.1 Hay, W.W.; Mohler, H.P.; Roth, P.H.; Schmidt, R.R.; Boudreaux, J.E. (1967), "Calcareous nannoplankton zonation of the Cenozoic of the Gulf Coast and Caribbean-Antillean area, and transoceanic correlation", Transactions of the Gulf Coast Association of Geological Societies 17: 428–480.
- ↑ Schaechter, Moselio (2012). Eukaryotic Microbes. Academic Press. p. 239. ISBN 978-0-12-383876-6. Retrieved 30 January 2015.
- ↑ "Biogeography and dispersal of micro-organisms: a review emphasizing protists.", Acta Protozoologica 45 (2), 2005: 111–136
- ↑ Buitenhuis, Erik T.; Pangerc, Tanja; Franklin, Daniel J.; Le Quéré, Corinne; Malin, Gill (2008), "Growth Rates of Six Coccolithoripd Strains as a Function of Temperature", Limnology and Oceanography 53 (3): 1181–1185, doi:10.4319/lo.2008.53.3.1181
- ↑ Egge, JK; Aksnes, DL (1992), "Silicate as regulating nutrient in phytoplankton competition", Marine Ecology Progress Series 83 (2): 281–289, doi:10.3354/meps083281
- ↑ Bentaleb, I. et al. (1999), "Silicate as regulating nutrient in phytoplankton competition", Marine Chemistry 64 (4): 301–313.
- ↑ 9.0 9.1 Smith, H.E.K. et al. (2012), "Predominance of heavily calcified coccolithophores at low CaCO3 saturation during winter in the Bay of Biscay", Proceedings of the National Academy of Sciences 109 (23): 8845–8849, doi:10.1073/pnas.1117508109
- ↑ 10.0 10.1 Yunev, O.A. et al. (2007), "Nutrient and phytoplankton trends on the western Black Sea shelf in response to cultural eutrophication and climate changes", Estuarine, Coastal, and Shelf Science 74: 63–67, doi:10.1016/j.ecss.2007.03.030
- ↑ 11.0 11.1 Moheimani, N.R.; Webb, J.P.; Borowitzka, M.A. (2012), "Bioremediation and other potential applications of coccolithophorid algae: A review. . Bioremediation and other potential applications of coccolithophorid algae: A review", Algal Research 1 (2): 120–133, doi:10.1016/j.algal.2012.06.002
- ↑ Falkowski, P.G.; Knoll, A.H. (August 29, 2007). Evolution of Primary Producers in the Sea. Amsterdam, Boston: Elsevier Academic Press. ISBN 9780123705181.
- ↑ 13.0 13.1 13.2 Hogan, M.C. ""Coccolithophores"". In Cleveland, Cutler J. Encyclopedia of Earth. Washington, D.C.: Environmental Information Coalition, National Council for Science and the Environment.
- ↑ Linschooten, Cornelis et al. (1991), "Role of the light-dark cycle and medium composition on the production of coccoliths by Emiliania huxleyi (haptophyceae)", Journal of Phycology 27 (1): 82–86, doi:10.1111/j.0022-3646.1991.00082.x
- ↑ 15.0 15.1 15.2 de Vargas, C.; Aubrey, M.P.; Probert, I.; Young, J. (2007). "From coastal hunters to oceanic farmers.". In Falkowski, P.G.; Knoll, A.H. Origin and Evolution of Coccolithophores. Boston: Elsevier. pp. 251–285.
- ↑ 16.0 16.1 Young, J.R.; Karen, H. (2003). "Biomineralization Within Vesicles: The Calcite of Coccoliths". In Dove, P.M.; Yoreo, J.J.; Weiner, S. Reviews in Mineralogy and Geochemistry. Washington, D.C.: Mineralogical Socity of America. pp. 189–216.
- ↑ 17.0 17.1 17.2 Young, J.R. et al. (2009), "Coccolith function and morphogenesis: insights from appendage-bearing coccolithophores of the family syracosphaeraceae (haptophyta)", Journal of Phycology 45 (1): 213–226, doi:10.1111/j.1529-8817.2008.00643.x
- ↑ Westbroek, P. et al. (1983), "Calcification in Coccolithophoridae: Wasteful or Functional?", Ecological Bulletins: 291–299
- ↑ 19.0 19.1 Jordan, R.W. (2012), "Haptophyta", eLS, doi:10.1002/9780470015902.a0001981.pub2
- ↑ Irie, Takahiro et al. (2010), "Increasing costs due to ocean acidification drives phytoplankton to be more heavily calcified: optimal growth strategy of coccolithophores", PloS One 5 (10): e13436, doi:10.1371/journal.pone.0013436
- ↑ 21.0 21.1 Billard, Chantal; Inouye, Isoa (August 17, 2004). "What is new in coccolithophore biology?". In Thierstein, Hans R.; Young, Jeremy R. Coccolithophores-from molecular processes to global impact. Berlin: Springler. pp. 1–29. ISBN 9783540219286..
- ↑ 22.0 22.1 Vardi, A. et al. (2012), "Host–virus dynamics and subcellular controls of cell fate in a natural coccolithophore population", Proceedings of the National Academy of Sciences 109 (47): 19327–19332, doi:10.1073/pnas.1208895109
- ↑ 23.0 23.1 23.2 Houdan; Probert, I; Zatylny, C; Véron, B; Billard, C et al. (2006), ". Ecology of oceanic coccolithophores. I. Nutritional preferences of the two stages in the life cycle of Coccolithus braarudii and Calcidiscus leptoporus", Aquatic Microbial Ecology 44: 291–301, doi:10.3354/ame044291
- ↑ 24.0 24.1 Geisen, M. et al. (August 17, 2004). "Species level variation in coccolithophores=". In Thierstein, Hans R.; Young, Jeremy R. Coccolithophores-from molecular processes to global impact. Berlin: Springler. pp. 1–29. ISBN 9783540219286..
- ↑ 25.0 25.1 25.2 25.3 Jordan, R. W.; Chamberlain, A.H.L. (1997), "Biodiversity among haptophyte algae", Biodiversity & Conservation 6 (1): 131–152
- ↑ Boeckel; Baumann, Karl-Heinz; Henrich, Rüdiger; Kinkel, Hanno et al. (2006), "Coccolith distribution patterns in South Atlantic and Southern Ocean surface sediments in relation to environmental gradients", Deep Sea Research Part I:Oceanographic Research Papers 53 (6): 1073–1099, doi:10.1016/j.dsr.2005.11.006
- ↑ 27.0 27.1 27.2 Okada; Honjo, Susumu et al. (1973), "The distribution of oceanic coccolithophores in the Pacific", Deep Sea Research and Oceanographic Abstracts 20 (4): 355–374, doi:10.1016/0011-7471(73)90059-4
- ↑ 28.0 28.1 Kinkel, H. et al. (2000), "Coccolithophores in the equatorial Atlantic Ocean: response to seasonal and Late Quaternary surface water variability", Marine Micropaleontology 39 (1–4): 87–112, doi:10.1016/s0377-8398(00)00016-5
- ↑ Rost, B.; Riebesell, U. (2004), "Coccolithophores and the biological pump: responses to environmental changes", Coccolithophores 2: 99–125, doi:10.1007/978-3-662-06278-4_5, ISBN 978-3-642-06016-8
- ↑ Morrissey, J.F.; Sumich, J.L. (2012). Introduction to the Biology of Marine Life. p. 67.
- ↑ 31.0 31.1 Houdan, A. et al. (2004), "Toxicity of coastal coccolithophores (Prymnesiophyceae, Haptophyta)", Journal of plankton research 26 (8): 875–883, doi:10.1093/plankt/fbh079
- ↑ Litchman, E. et al. (2007), "The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level", Ecology Letters 10 (12): 1170–1181, doi:10.1111/j.1461-0248.2007.01117.x, PMID 17927770
- ↑ Frada, M. et al. (2008), "The "Cheshire Cat" escape strategy of the coccolithophore Emiliania huxleyi in response to viral infection", Proceedings of the National Academy of Sciences 105 (41): 15944–15949, doi:10.1073/pnas.0807707105
- ↑ Mejia, R. (2011), "Will Ion Channels Help Coccolithophores Adapt to Ocean Acidification?", PLoS Biology 9 (6): e1001087, doi:10.1371/journal.pbio.1001087, PMC 3119655, PMID 21713029
- ↑ Mackinder; Wheeler, Glen; Schroeder, Declan; Riebesell, Ulf; Brownlee, Colin et al. (2010), "Molecular Mechanisms Underlying Calcification in Coccolithophores", Geomicrobiology Journal 27 (6–7): 585–595, doi:10.1080/01490451003703014
- ↑ Bates; Michaels, Anthony F.; Knap, Anthony H. et al. (1996), "Alkalinity changes in the Sargasso Sea; geochemical evidence of calfication?", Marine Chemistry 51 (4): 347–358, doi:10.1016/0304-4203(95)00068-2
- ↑ 37.0 37.1 Marsh, M.E. (2003), "Regulation of CaCO3 formation in coccolithophores", Comparative Biochemistry and Physiology Part B 136 (4): 743–754, doi:10.1016/s1096-4959(03)00180-5
- ↑ 38.0 38.1 Beaufort, L. et al. (2011), "Sensitivity of coccolithophores to carbonate chemistry and ocean acidification", Nature 476 (7358): 80, doi:10.1038/nature10295, PMID 21814280
- ↑ Tyrell, T. et al. (1999), "Optical impacts of oceanic coccolithophore blooms", Journal of Geophysical Research 104: 3223–3241, Bibcode:1999JGR...104.3223T, doi:10.1029/1998jc900052
- ↑ "cal.mar.o".
- ↑ Self-Trail, J.M. et al. (2012), "Calcareous Nannofossil Assemblage Changes Across the Paleocene-Eocene Thermal Maximum: Evidence from a Shelf Setting", Marine Micropaleontology, 92-93: 61–80, doi:10.1016/j.marmicro.2012.05.003
- ↑ Lloyd, G.T. et al. (2011), "Quantifying the deep-sea rock and fossil record bias using coccolithophores", Geological Society, London, Special Publications 358 (1): 167–177, doi:10.1144/sp358.11
- ↑ http://www.norge-bank.no
External links
Sources of detailed information
- Nannotax3 – illustrated guide to the taxonomy of coccolithophores and other nannofossils.
- INA — International Nannoplankton Association
- Emiliania huxleyi Home Page
Introductions to coccolithophores
- University of California, Berkeley. Museum of Paleontology: "Introduction to the Prymnesiophyta".
- The Paleontology Portal: Calcareous Nanoplankton
- RadioLab – podcast on coccolithophores
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
