Cobalt-factor II C20-methyltransferase
| cobalt-factor II C20-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.1.1.151 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a cobalt-factor II C20-methyltransferase (EC 2.1.1.151) is an enzyme that catalyzes the chemical reaction
- S-adenosyl-L-methionine + cobalt-factor II
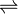 S-adenosyl-L-homocysteine + cobalt-factor III
S-adenosyl-L-homocysteine + cobalt-factor III
Thus, the two substrates of this enzyme are S-adenosyl methionine and cobalt-factor II, whereas its two products are S-adenosylhomocysteine and cobalt-factor III.
This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:cobalt-factor-II C20-methyltransferase. This enzyme is also called CbiL. This enzyme participates in porphyrin and chlorophyll metabolism.
References
- Spencer P, Stolowich NJ, Sumner LW, Scott AI (1998). "Definition of the redox states of cobalt-precorrinoids: investigation of the substrate and redox specificity of CbiL from Salmonella typhimurium". Biochemistry. 37 (42): 14917–27. doi:10.1021/bi981366f. PMID 9778368.