Circulene

A circulene is a macrocyclic arene in which a central n-sided polygon is completely surrounded and fused by benzenoids.[1] [5]circulene (corannulene), [6]circulene (coronene), [7]circulene,[2][3][4][5] and [12]circulene (kekulene) have all been synthesized in the laboratory. These compounds belong to a larger class of geodesic polyarenes. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle-shaped structure (compare to cones and partial cones in calixarenes). Conceptually related compounds are the helicenes.
Quadrannulene
The isolation of quadrannulene or tetrabenzo[4]circulene has been reported.[6]
[8]Circulenes
The isolation of the [8]circulene derivative 2,5,6,9,10,13,14-octamethyl-3,4,7,8,11,12,15,16-octa(4-tolyl)[8]circulene has been reported [7]
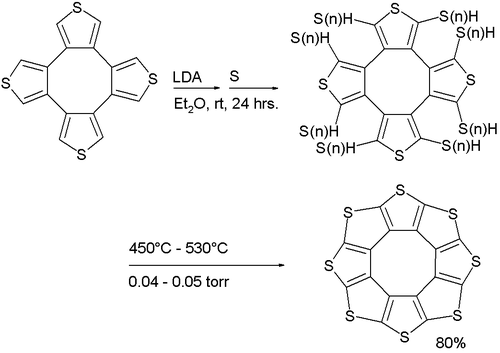
Sulflower
A stable octacirculene based on thiophene, not containing any hydrogen, has been reported with the name sulflower (a portmanteau of sulfur and sunflower). With molecular formula (C2S)8 the compound is considered an analogue of carbon sulfide. The molecule is flat and together with the 9-membered homologue is at a local strain energy minimum.[8]
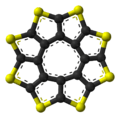 |  |  | 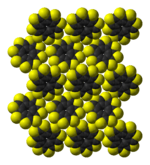 |
the sulflower molecule | the sulflower molecule | molecules in the crystal structure | molecules in the crystal structure |
Its synthesis (a variation of the Ferrario reaction) is based on deprotonation of a tetrathiophene with lithium diisopropylamide followed by reaction with elemental sulfur to a sulfur-substituted intermediate followed by vacuum pyrolysis.
Sulflower molecules have a perfect planar structure with high symmetry (D8h). Because of their planar structure, they are predicted to store a lot of hydrogen molecules between the stacks. The conformation of the H2 molecule is calculated to be "standing up" over the five membered rings. Detailed DFT calculations have been performed on these molecules.[9]
Gallery
-
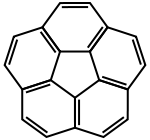
Corannulene ([5]circulene)
-
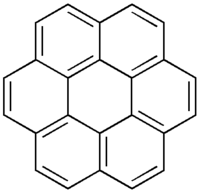
Coronene ([6]circulene)
-

[7]circulene
-

Sulflower, an octacirculene
-

Kekulene ([12]circulene)
References
- ↑ J.H. Dopper, Hans Wynberg, Heterocirculenes a new class of polycyclic aromatic hydrocarbons, Tetrahedron Letters, Volume 13, Issue 9, 1972, Pages 763-766, ISSN 0040-4039, doi:10.1016/S0040-4039(01)84432-4.
- ↑ Synthesis and characterization of [7]circulene Koji Yamamoto, Tadashi Harada, Masao Nakazaki, Takuo Naka, Yasushi Kai, Shigeharu Harada, and Nobutami Kasai Journal of the American Chemical Society 1983 105 (24), 7171-7172 doi:10.1021/ja00362a025
- ↑ Synthesis and molecular structure of [7]circulene Koji. Yamamoto, Tadashi. Harada, Yoshio. Okamoto, Hiroaki. Chikamatsu, Masao. Nakazaki, Yasushi. Kai, Takuo. Nakao, Mitsuya. Tanaka, Shigeharu. Harada, and Nobutami. Kasai Journal of the American Chemical Society 1988 110 (11), 3578-3584 doi:10.1021/ja00219a036
- ↑ Yamamoto, K., Sonobe, H., Matsubara, H., Sato, M., Okamoto, S. and Kitaura, K. (1996), Convenient New Synthesis of [7]Circulene. Angew. Chem. Int. Ed. Engl., 35: 69–70. doi:10.1002/anie.199600691
- ↑ Extended systems of closed helicene. Synthesis and characterization of [7] and [7.7]-circulene Koji Yamamoto Pure & Appl. Chem., Vol. 65, No. 1, pp. 157-163, 1993. Online article
- ↑ Bharat, Bhola, R., Bally, T., Valente, A., Cyrański, Michał K., Dobrzycki, Ł., Spain, Stephen M., Rempała, P., Chin, Matthew R. and King, Benjamin T. (2010), Quadrannulene: A Nonclassical Fullerene Fragment. Angew. Chem. Int. Ed., 49: 399–402. doi:10.1002/anie.200905633
- ↑ Feng, C.-N., Kuo, M.-Y. and Wu, Y.-T. (2013), Synthesis, Structural Analysis, and Properties of [8]Circulenes . Angew. Chem. Int. Ed., 52: 7791–7794. doi:10.1002/anie.201303875
- ↑ Communication Sulflower: A New Form of Carbon Sulfide Konstantin Yu. Chernichenko, Viktor V. Sumerin, Roman V. Shpanchenko, Elizabeth S. Balenkova, Valentine G. Nenajdenko Angewandte Chemie International Edition Volume 45, Issue 44 , Pages 7367 - 7370 2006 doi:10.1002/anie.200602190
- ↑ Computational design of high hydrogen adsorption efficiency in molecular “Sulflower” Ayan Datta, Swapan K. Pati, J. Phys. Chem. C (Letter) 111, 4487 (2007).
| ||||||||||||||||||||||