Chromium(VI) oxide peroxide
 | |
| Names | |
|---|---|
| Other names
chromium(VI) oxide peroxide | |
| Identifiers | |
| |
| Jmol-3D images | Image |
| |
| Properties | |
| Molecular formula |
CrO5 |
| Molar mass | 131.99 g·mol−1 |
| soluble (decomposes without stabilisers) | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Chromium(VI) peroxide (CrO5) or chromium oxide peroxide is an unstable compound formed by the addition of acidified hydrogen peroxide solutions to solutions of metal chromates, such as sodium chromate. The generally yellow chromates turn to dark blue-brown as chromium(VI) peroxide is formed. The metal chromate reacts with hydrogen peroxide and an acid to give chromium peroxide, water, and the metal salt of the acid.
- M2CrO4 + 2 H2O2 + 2 H+ → CrO5 + 3 H2O + 2 M+
After a few seconds, the chromium(VI) peroxide decomposes to turn green as chromium(III) compounds are formed.[1] To avoid this decomposition, it is possible to stabilize chromium(VI) oxide peroxide in a water-immiscible organic solvent such as diethyl ether, butan-1-ol or amyl acetate by adding a layer of the organic solvent above the chromate/dichromate solution and shaking during the addition of hydrogen peroxide. In this way, the chromium(VI) peroxide (unstable in the aqueous phase in which newly formed) is dissolved in the immiscible organic solvent. In this condition it can be observed over a much longer period.
- 2 CrO5 + 7 H2O2 + 6 H+ → 2 Cr3+ + 10 H2O + 7 O2
This compound contains one oxo ligand and two peroxo ligands, making a total of five oxygen atoms per chromium atom.
Derivatives
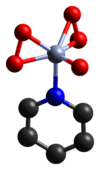
The etherate, bipyridyl and pyridyl complexes of this compound have been found to be effective oxidants in organic chemistry.[2] The structure of the pyridyl complex has been determined crystallographically.[3]
Gallery
-

An aqueous solution of chromium peroxide
-

A very dilute solution of chromium peroxide
-
_oxide_peroxide_(stabilized_in_ether_phase).jpg)
chromium(VI) oxide peroxide stabilized in ether phase (above) and chromium(III) aqueous solution (below).
References
- ↑ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils; (1985). "Chromium" (in German). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 1081–1095. ISBN 3-11-007511-3."
- ↑ Firouzabadi, H.; Iranpoor, N.; Kiaeezadeh, F.; Toofan, J. (1986). "Chromium(VI) based oxidants-1 Chromium peroxide complexes as versatile, mild, and efficient oxidants in organic synthesis". Tetrahedron 42: 719. doi:10.1016/S0040-4020(01)87476-7.
- ↑ Stomberg, Rolf (1962). "Crystal Structure of Peroxochromates, CrO5⋅C5H5N". Nature 196 (4854): 570. doi:10.1038/196570b0.
External links
- Experimental details and photo (German)
| ||||||||||||||||||||||||||||||||||