Chichibabin reaction
The Chichibabin reaction (pronounced ' (chē')-chē-bā-bēn) is a method for producing 2-aminopyridine derivatives by the reaction of pyridine with sodium amide. It was reported by Aleksei Chichibabin in 1914.[1] The following is the overall form of the general reaction:
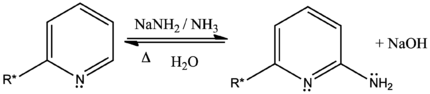
The direct amination of pyridine with sodium amide takes place in liquid ammonia. Following the addition elimination mechanism first a nucleophilic NH2− is added while a hydride (H−) is leaving.
Ciganek describes an example of an intramolecular Chichibabin reaction in which a nitrile group on a fused ring is the source of nitrogen in amination.[2]
Mechanism

It is widely accepted that the Chichibabin reaction mechanism is an addition-elimination reaction that proceeds through an σ-adduct (Meisenheimer adduct) intermediate (the third structure).[3] First, the nucleophilic NH2− group adds to the δ+ ring carbon pushing electrons onto the ring nitrogen and forming the anionic σ-adduct, which is stabilized by sodium. Electrons from the nitrogen are then pushed towards the ring forming a C=N bond and kicking out a hydride ion. The hydride ion abstracts a hydrogen from the positively charged nitrogen, forming hydrogen gas. The ring nitrogen then pushes electrons back into the ring, regaining aromaticity, the now negatively charged NH group abstracts a proton from water giving us the product.
Reaction progress can be measured by the formation of hydrogen gas and red color from σ-adduct formation.[3] Sodium amide is a handy reagent for the Chichibabin reaction but handling it can be dangerous and caution is advised.[4]
- σ-adduct (Meisenheimer adduct) formation
Evidence indicates that before addition of the amino group, the ring nitrogen is sorbed onto the surface of sodium amide and the sodium cation forms a coordination complex.[3] This increases the δ+ on the α-carbon, thus 1,2-addition of sodium amide is favored over 1,4-addition. The proximity of the amino group to the α-carbon once the coordination complex is formed also makes the 1,2-addition more likely to occur.
Some data exists that supports a single electron transfer as the proposed pathway for σ-adduct formation.[3]
In most cases, the anionic σ-adduct is unstable making its formation the rate determining step.[3]
- Hydride ion elimination
In addition to the mechanism shown above, other pathways have been proposed for the elimination step.[3] The mechanism above, loss of the hydride ion followed by abstraction of a proton, is supported by the fact that the nucleophile needs at least one hydrogen atom for the reaction to proceed. Another competing pathway could be the elimination of hydride by sodium to form sodium hydride.
Factors influencing reaction
Different aromatic nitrogen heterocyclic compounds proceed through the Chichibabin reaction in a matter of minutes and others can take hours. Factors that influence the reaction rate include:
- Basicity - The ideal pKa range is 5-8 and the reaction either does not proceed, or proceeds poorly outside of this range. The reaction occurs faster under more basic conditions but only up to a point because when electron density builds up on the α-carbon, it makes it less electrophilic. The strongest base known to aminate is 4-dimethylaminopyridine (pKa 9.37).[3]
- δ+ on α-carbon - For kinetically controlled additions, the rate of amination is related to the magnitude of the partial positive charge on the carbon next to the ring nitrogen. For thermodynamically controlled additions, the rate of amination is related to the stability of the σ-adduct.[3]
- Ease of hydride elimination - Success of this reaction is also dependent on the ease at which the hydride ion leaves and the ring regains aromaticity. The rate of amination for three azoles proceeds quickest to slowest as follows: 1-methylbenzimidazole > 1-methylnaphth-[2,3-d]imidazole > 3-methylnaphth[1,2-d]imidazole.[3] Since the addition of the amide ion proceeds quickly with these substrates, the differences in reaction rates is most likely their propensity for hydride elimination and reformation of an aromatic ring.[3]
- Substituents - Electron-withdrawing groups inhibit the Chichibabin reaction. Three proposed ideas of why this is are (1) they decrease the basicity of the ring nitrogen and slow down the sorption on sodium amide, (2) these electron-withdrawing groups can also form complexes with sodium amide, and (3) for single electron transfer pathway, altering the distribution of spin density of the intermediate radical anion.[3]
Substrates with σ-dimethoxy groups don't aminate because they form a stable complex with sodium amide.[3]
Electron-donating groups also inhibit the Chichibabin reaction because of their deactivating effects.[3]
- Benzo annelation - Since the hydride ion is a poor leaving group, benzo annelation increases reactivity of the substrate in the Chichibabin reaction. This is demonstrated by the fact that 1-methylimidazole does not work as a substrate, but 1-methylbenzimidazole reacts easily.[3]
- Solvent - The ability of the polar anionic σ-adduct to form will depend on the solvating capacity and the dielectric constant of the solvent.[3]
- Temperature - The rule of thumb in aprotic solvents (where σ-adduct formation is the rate determining step) is to run the reaction at the lowest temperature for good hydrogen evolution to avoid the decomposition that occurs at high temperatures.
Side reaction
Dimerization is a side reaction that can occur. When heated in xylene and sodium amide at atmospheric pressure, the substrate 4-tert-butylpyridine produces 89% of the dimer product (4,4'-di-tert-butyl-2,2'-bipyridine) and only 11% of the aminated Chichibabin product (2-amino-4-tert-butylpyridine).[3] When subjected to 350 psi nitrogen pressure and the same conditions, the yields are 74% of the aminated Chichibabin product and 26% of the dimer product.[3]
See also
References
- ↑ Chichibabin, A. E.; Zeide, O. A. (1914). "[New Reaction for Compounds Containing the Pyridine Nucleus]". Zhur. Russ. Fiz. Khim. Obshch (J. Russ. Phys. Chem. Soc.) 46: 1216–36.
- ↑ Ciganek, E. (1992). "Tertiary Carbinamines by Addition of Organocerium Reagents to Nitriles and Ketimines". Journal of Organic Chemistry 57 (16): 4521–7. doi:10.1021/jo00042a037.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 3.10 3.11 3.12 3.13 3.14 3.15 3.16 McGill, C.; Rappa, A. (1988). "Advances in the Chichibabin Reaction". Advances in Heterocyclic Chemistry 44: 1–79. doi:10.1016/s0065-2725(08)60261-5.
- ↑ Shreve, R. N.; Riechers, E. H.; Rubenkoenig, H.; Goodman, A. H. (1940). "Amination in the Heterocyclic Series by Sodium Amide". Industrial and Engineering Chemistry 32 (2): 173–8. doi:10.1021/ie50362a008.