Cerium(IV) oxide
_oxide.jpg) | |
 | |
| Names | |
|---|---|
| IUPAC name
Cerium(IV) oxide | |
| Other names
Ceric oxide, Ceria, Cerium dioxide | |
| Identifiers | |
| 1306-38-3 12014-56-1 (monohydrate) | |
| ChEBI | CHEBI:79089 |
| ChemSpider | 8395107 |
| |
| Jmol-3D images | Image |
| PubChem | 73963 |
| |
| UNII | 619G5K328Y |
| Properties | |
| CeO2 | |
| Molar mass | 172.115 g/mol |
| Appearance | white or pale yellow solid, slightly hygroscopic |
| Density | 7.215 g/cm3 |
| Melting point | 2,400 °C (4,350 °F; 2,670 K) |
| Boiling point | 3,500 °C (6,330 °F; 3,770 K) |
| insoluble | |
| Structure | |
| Crystal structure | cubic (fluorite)[1] |
| Hazards | |
| NFPA 704 | |
| Related compounds | |
| Related compounds |
Cerium(III) oxide |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Cerium(IV) oxide, also known as ceric oxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2.
Cerium(IV) oxide is formed by the calcination of cerium oxalate or cerium hydroxide.
Powdered ceria is slightly hygroscopic and will also absorb a small amount of carbon dioxide from the atmosphere.[2]
Cerium also forms cerium(III) oxide, Ce
2O
3, which is an unstable compound that will oxidize to cerium (IV) oxide under standard conditions for temperature and pressure.[3]
Structure
Cerium oxide has the fluorite structure, space group Fm3m, #225 containing 8 coordinate Ce4+ and 4 coordinate O2− . At high temperatures it can be reduced to a non-stoichiometric, anion deficient form that retains the fluorite lattice, CeO(2-x) where 0 < 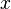 < 0.28 [4]. The value of
< 0.28 [4]. The value of 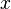 depends on both the temperature and the oxygen partial pressure. The equation
depends on both the temperature and the oxygen partial pressure. The equation
has been shown to predict the equilibrium non stoichiometry 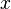 over a wide range of oxygen partial pressures (103 - 10-4 Pa) and temperatures (1000-1900 °C) [5].
over a wide range of oxygen partial pressures (103 - 10-4 Pa) and temperatures (1000-1900 °C) [5].
The non stoichiometric form has a blue to black color, and exhibits both ionic and electronic conduction with ionic being the most significant at temperatures > 500 °C. [6]
Applications
Cerium(IV) oxide is used in ceramics, to sensitize photosensitive glass, as a catalyst and as a catalyst support, to polish glass and stones, in lapidary as an alternative to "jeweller's rouge". It is also known as "optician's rouge".[7]
It is also used in the walls of self-cleaning ovens as a hydrocarbon oxidation catalyst during the high-temperature cleaning process.
While it is transparent for visible light, it absorbs ultraviolet radiation strongly, so it is a prospective replacement of zinc oxide and titanium dioxide in sunscreens, as it has lower photocatalytic activity.[8] However, its thermal catalytic properties have to be decreased by coating the particles with amorphous silica or boron nitride. The use of these nanoparticles, which can penetrate the body and reach internal organs, has been criticized as unsafe.[9]
Cerium oxide has found use in infrared filters, as an oxidizing species in catalytic converters and as a replacement for thorium dioxide in incandescent mantles[10]
Fuel cell electrolyte
In the doped form (it comes from cerium and oxygen), ceria is of interest as a material for solid oxide fuel cells or SOFCs because of its relatively high oxygen ion conductivity (i.e. oxygen atoms readily move through it) at intermediate temperatures (500–650 °C). Undoped and doped ceria also exhibit high electronic conductivity at low partial pressures of oxygen due to reduction of the cerium ion leading to the formation of small polarons. However, doped ceria has an extended electrolytic region (area of predominant ionic conductivity), over that of ceria, that allows its use as an electrolyte in SOFCs below 600-650 °C. Exposure to hydrogen at high temperature (800 °C) has been shown to cause significant damage to the grain boundaries leading to cracking. Exposure to other reducing agents such as carbon monoxide is less damaging.[11] Substituting a fraction of the ceria with gadolinium (as in Gadolinium doped ceria) or samarium will introduce oxygen vacancies in the crystal without adding electronic charge carriers. This increases the ionic conductivity and results in a better electrolyte.
Under reducing conditions, such as those experienced on the anode side of the fuel cell, a large amount of oxygen vacancies within the ceria electrolyte can be formed. Some of the cerium(IV) oxide is also reduced to cerium(III) oxide under these conditions, which consequently increases the electronic conductivity of the material.[11] The lattice constant of ceria increases under reducing conditions as well as with decreasing nanocrystal size in nanocrystalline ceria, as a result of reduction of the cerium cation from a 4+ to a 3+ state in order to charge compensate for oxygen vacancy formation.[12]
Catalyst
Ceria has been used in catalytic converters in automotive applications. Since ceria can become non-stoichiometric in oxygen content (i.e. it can give up oxygen without decomposing) depending on its ambient partial pressure of oxygen, it can release or take in oxygen in the exhaust stream of a combustion engine. In association with other catalysts, ceria can effectively reduce NOx emissions as well as convert harmful carbon monoxide to the less harmful carbon dioxide. Ceria is particularly interesting for catalytic conversion economically because it has been shown that adding comparatively inexpensive ceria can allow for substantial reductions in the amount of platinum needed for complete oxidation of NOx and other harmful products of incomplete combustion.
Due to its fluorite structure, the oxygen atoms in a ceria crystal are all in a plane with one another, allowing for rapid diffusion as a function of the number of oxygen vacancies. As the number of vacancies increases, the ease at which oxygen can move around in the crystal increases, allowing the ceria to reduce and oxidize molecules or co-catalysts on its surface. It has been shown that the catalytic activity of ceria is directly related to the number of oxygen vacancies in the crystal, frequently measured by using X-ray photoelectron spectroscopy to compare the ratios of Ce3+
to Ce4+
in the crystal.
Ceria can also be used as a co-catalyst in a number of reactions, including the water-gas shift reaction[13] and steam reforming of ethanol or diesel fuel into hydrogen gas and carbon dioxide (with varying combinations of rhodium oxide, iron oxide, cobalt oxide, nickel oxide, platinum, and gold), the Fischer-Tropsch reaction, and selected oxidation (particularly with lanthanum). In each case, it has been shown that increasing the ceria oxygen defect concentration will result in increased catalytic activity, making it very interesting as a nanocrystalline co-catalyst due to the heightened number of oxygen defects as crystallite size decreases—at very small sizes, as many as 10% of the oxygen sites in the fluorite structure crystallites will be vacancies, resulting in exceptionally high diffusion rates.
Water splitting
The cerium(IV) oxide–cerium(III) oxide cycle or CeO2/Ce2O3 cycle is a two step thermochemical water splitting process based on cerium(IV) oxide and cerium(III) oxide for hydrogen production.[14]
Anti-oxidant
Uniform 3.8-nanometer spheres of cerium oxide with a thin coating of fatty oleic acid make them biocompatible. The spheres act as anti-oxidants, absorbing ROS free radicals. One gram of these nanoparticles can have the surface area of a football field. Potential uses include treatments for traumatic brain injury, cardiac arrest, Alzheimer’s disease and could help to reduce palliating radiation-induced side effects suffered by cancer patients. The nanoparticles also have potential to protect astronauts from long-term exposure to radiation in space and perhaps even slow the effects of aging.[15]
The particles continue to work over time by reverting to their initial state, for unexplained reasons.[15] It is suggested that a flip-flop mechanism between oxidation states of the Ce atom is involved. This feature differentiates cerium oxide from other inorganic anti-oxidants.[16]
The coating is thin enough to let oxygen pass through to the particle, but robust enough to protect it through many cycles of ROS absorption. The particles are uniform with well-defined surfaces. They are made without water, which maximizes the surface gaps available for oxygen scavenging.[15][17]
Defects
In the most stable fluorite phase of ceria, it exhibits several defects depending on partial pressure of oxygen or stress state of the material.[18][19] The primary defects of concern are oxygen vacancies and small polarons (electrons localized on cerium cations) because these two are located in the "useful" range of ceria. In the case of oxygen defects, the increased diffusion rate of oxygen in the lattice causes increased catalytic activity as well as an increase in ionic conductivity, making ceria interesting as a fuel cell electrolyte in solid-oxide fuel cells.
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ Robert David Green Carbon dioxide reduction on gadolinium-doped ceria cathodes. PhD Thesis. Department of Chemical Engineering, CASE Western Reserve University. May, 2009
- ↑ Thermodynamic data
- ↑ Defects and Defect Processes in Nonmetallic Solids By William Hayes, A. M. Stoneham Courier Dover Publications, 2004
- ↑ Bulfin, B.; Lowe, A. J.; Keogh, K. A.; Murphy, B. E.; Lübben, O.; Krasnikov, S. A.; Shvets, I. V. (2013). "Analytical Model of CeO2Oxidation and Reduction". The Journal of Physical Chemistry C 117 (46): 24129. doi:10.1021/jp406578z.
- ↑ Ghillanyova, K; Galusek, D (2011). "Chapter 1: Ceramic oxides". In Riedel, Ralf; Chen, I-Wie. Ceramics Science and Technology, Materials and Properties, vol 2. John Wiley & Sons. ISBN 978-3-527-31156-9.
- ↑ Properties of Common Abrasives (Boston Museum of Fine Arts)
- ↑ Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B.; Shaporev, A.S.; Polezhaeva, O.S.; Baranchikov, A.Ye.; Spivak, N.Ya.; Tretyakov, Yu.D. (2011). "UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions". Journal of Photochemistry and Photobiology B: Biology 102 (1): 32–38. doi:10.1016/j.jphotobiol.2010.09.002.
- ↑ "Suncream may be linked to Alzheimer's disease, say experts". Daily Mail (London). 24 August 2009. Retrieved 2009-08-25.
- ↑ Cerium dioxide. nanopartikel.info
- ↑ 11.0 11.1 Badwal, SPS (2013). "Structural and Microstructural Stability of Ceria–Gadolinia Electrolyte Exposed to Reducing Environments of High Temperature Fuel Cells". J. Mater. Chem. A 1 (36): 10768. doi:10.1039/c3ta11752a.
- ↑ Deshpande, Sameer; Patil, Swanand; Kuchibhatla, Satyanarayana VNT; Seal, Sudipta (2005). "Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide". Applied Physics Letters 87 (13): 133113. doi:10.1063/1.2061873.
- ↑ Jain, Rishabh (5 April 2014). "Synthesis of nano-Pt onto ceria support as catalyst for water–gas shift reaction by Reactive Spray Deposition Technology". Applied Catalysis A: General 475: 461–468. doi:10.1016/j.apcata.2014.01.053. Retrieved 13 September 2014.
- ↑ Hydrogen production from solar thermochemical water splitting cycles. solarpaces.org
- ↑ 15.0 15.1 15.2 "A super antioxidant based on material used in vehicle catalytic converters". KurzweilAI. 2013-10-14. Retrieved 2013-10-24.
- ↑ . doi:10.1039/c2ra20921g. Missing or empty
|title=(help) - ↑ Lee, S. S.; Song, W.; Cho, M.; Puppala, H. L.; Nguyen, P.; Zhu, H.; Segatori, L.; Colvin, V. L. (2013). "Antioxidant Properties of Cerium Oxide Nanocrystals as a Function of Nanocrystal Diameter and Surface Coating". ACS Nano: 131021104753002. doi:10.1021/nn4026806.
- ↑ Munnings,, C; SPS Badwal; D Fini (2014). "Spontaneous stress-induced oxidation of Ce ions in Gd-doped ceria at room temperature". Ionics 20 (8): 1117–1126. doi:10.1007/s11581-014-1079-2.
- ↑ Badwal, SPS; Daniel Fini; Fabio Ciacchi; Christopher Munnings; Justin Kimpton; John Drennan (2013). "Structural and microstructural stability of ceria – gadolinia electrolyte exposed to reducing environments of high temperature fuel cells". J. Mater. Chem. A 1 (36): 10768. doi:10.1039/C3TA11752A.
External links
| Wikimedia Commons has media related to Cerium(IV) oxide. |
| ||||||

![\Bigg( \frac{x}{0.35-x} \Bigg) = 106000\,\, [\mathrm{Pa}^{0.217}] \times P_{O_2}^{\,\,-0.217} \exp\Bigg( \frac{-195.6 \, \, [\mathrm{k\,J\,mol}^{-1}]}{RT} \Bigg)](../I/m/189338e8e5d6d43312ec533cd6bd33e8.png)