Cementite
| Steels and other iron–carbon alloy phases |
|---|
| Microstructures |
|
| Classes |
| Other iron-based materials |
|
Cementite, also known as iron carbide, is a chemical compound of iron and carbon, with the formula Fe3C (or Fe2C:Fe). By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure.[1] It is a hard, brittle material,[1] normally classified as a ceramic in its pure form, though it is more important in metallurgy.
Metallurgy

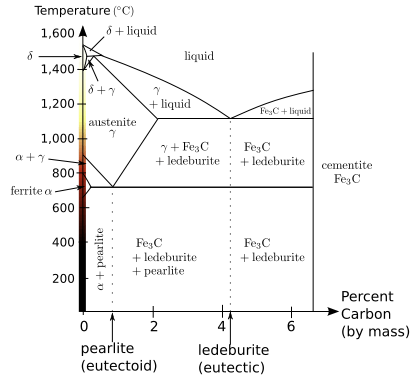
In the iron–carbon system (i.e. plain-carbon steels and cast irons) it is a common constituent because ferrite can contain at most 0.02wt% of uncombined carbon. Therefore, in carbon steels and cast irons that are slowly cooled a portion of the elements is in the form of cementite.[2] It forms directly from the melt in the case of white cast iron. In carbon steel, it either forms from austenite during cooling or from martensite during tempering. An intimate mixture with ferrite, the other product of austenite, forms a lamellar structure called pearlite.
Cementite is thermodynamically unstable, eventually being converted to ferrite and graphite.
Pure form
Cementite changes from ferromagnetic to paramagnetic at its Curie temperature of approximately 480 K.[3]

A natural iron carbide (containing minor amounts of nickel and cobalt) occurs in iron meteorites and is called cohenite after the German mineralogist Emil Cohen, who first described it.[4] As carbon is one of the possible minor light alloy components of metallic planetary cores, the high-pressure/high-temperature properties of cementite (Fe3C) as a simple proxy for cohenite are studied experimentally. The figure shows the compressional behaviour at room temperature.
Other iron carbides
Two other forms of metastable iron carbide have been identified in tempered steel. Epsilon (ε) carbide, hexagonal close-packed Fe2-3C, precipitates in plain-carbon steels of carbon content > 0.2%, tempered at 100-200°C. Non-stoichiometric ε-carbide dissolves above ~200°C, where Hägg carbides and cementite begin to form. Hagg carbide, monoclinic Fe5C2, precipitates in hardened tool steels tempered at 200-300°C.[5][6]
References
- ↑ 1.0 1.1 Smith & Hashemi 2006, p. 363.
- ↑ Smith & Hashemi 2006, pp. 366–372.
- ↑ S.W.J. Smith; W. White; S.G. Barker (1911). "The Magnetic Transition Temperature of Cementite". Proc. Phys. Soc. London 24 (1): 62–69. doi:10.1088/1478-7814/24/1/310.
- ↑ Vagn F. Buchwald, Handbook of Iron Meteorites, University of California Press 1975.
- ↑ G. Hägg, Z. Krist., Vol. 89, p 92-94, 1934.
- ↑ W.F. Smith, Structure and Properties of Engineering Alloys, McGraw-Hill Inc., 1981, p 61-62, ISBN 0-07-0585601.
Bibliography
- Smith, William F.; Hashemi, Javad (2006). Foundations of Materials Science and Engineering (4th ed.). McGraw-Hill. ISBN 0-07-295358-6.