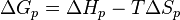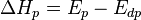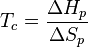Ceiling temperature
Ceiling temperature (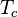 ) is a measure of the tendency of polymers to revert to their monomers. When a polymer is at its ceiling temperature, the rate of polymerization and depolymerization of the polymer are equal. Generally, the ceiling temperature of a given polymer is correlated to the steric hindrance of the polymer’s monomers. Polymers with high ceiling temperatures are often commercially useful.
) is a measure of the tendency of polymers to revert to their monomers. When a polymer is at its ceiling temperature, the rate of polymerization and depolymerization of the polymer are equal. Generally, the ceiling temperature of a given polymer is correlated to the steric hindrance of the polymer’s monomers. Polymers with high ceiling temperatures are often commercially useful.
Thermodynamics of polymerization
At constant temperature, the reversibility of polymerization can be determined using the Gibbs free energy equation:
where 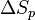 is the change of entropy during polymerization. The change of enthalpy during polymerization,
is the change of entropy during polymerization. The change of enthalpy during polymerization, 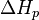 , is also known as the heat of polymerization, which is defined by
, is also known as the heat of polymerization, which is defined by
where 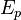 and
and 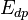 denote the activation energies for polymerization and depolymerization, respectively.
denote the activation energies for polymerization and depolymerization, respectively.
Entropy is the measure of randomness or chaos. A system has a lower entropy when there are few objects in the system and has a higher entropy when there are many objects in the system. Because the process of depolymerization involves a polymer being broken down into its monomers, depolymerization increases entropy. In the Gibbs free energy equation, the entropy term is negative. Enthalpy drives polymerizations. At low temperatures, the enthalpy term is greater than the 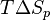 term, which allows polymerization to occur. At the ceiling temperature, the enthalpy term and the entropy term are equal. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer.[1] The ceiling temperature can be defined by
term, which allows polymerization to occur. At the ceiling temperature, the enthalpy term and the entropy term are equal. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer.[1] The ceiling temperature can be defined by
Monomer-polymer equilibrium
At the ceiling temperature, there will always be excess monomers in the polymer due to the equilibrium between polymerization and depolymerization. Polymers derived from simple vinyl monomers have such high ceiling temperatures that only a minuscule amount of monomers remain in the polymer at ordinary temperatures. The situation for α-methylstyrene, PhC(Me)=CH2, is an exception to this trend. Its ceiling temperature is around 66 °C. Steric hindrance is significant in polymers derived from α-methylstyrene because the phenyl and methyl groups are bonded to the same carbon. These steric effects in combination with stability of the tertiary benzylic α-methylstyryl radical give α-methylstyrene its relatively low ceiling temperature. When a polymer has a very high ceiling temperature, it degrades via bond cleavage reactions instead of depolymerization. A similar effect explains the relatively low ceiling temperature for poly(isobutylene).
Ceiling temperatures of common monomers
| Monomer | Ceiling Temperature (°C)[2] | Structure |
|---|---|---|
| 1,3-butadiene | 585 | CH2=CHCH=CH2 |
| ethylene | 610 | CH2=CH2 |
| isobutylene | 175 | CH2=CMe2 |
| isoprene | 466 | CH2=C(Me)CH=CH2 |
| methyl methacrylate | 198 | CH2=C(Me)CO2Me |
| α-methylstyrene | 66 | PhC(Me)=CH2 |
| styrene | 395 | PhCH=CH2 |
| tetrafluoroethylene | 1100 | CF2=CF2 |
References
- ↑ Carraher Jr; Charles E (2010). "7". Introduction of Polymer Chemistry (2nd ed.). New York: CRC Press, Taylor and Francis. p. 224. ISBN 978-1-4398-0953-2.
- ↑ Stevens, Malcolm P. (1999). "6". Polymer Chemistry an Introduction (3rd ed.). New York: Oxford University Press. pp. 193–194. ISBN 978-0-19-512444-6.