Ceftriaxone
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
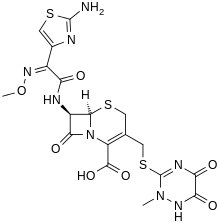
| (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)->2-(methoxyimino)acetyl]amino}-3-{[(2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-yl)thio]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Clinical data | |
| Trade names | Rocephin or Epicephin, Arixon, Elcefrin(LGls) |
| AHFS/Drugs.com | monograph |
| |
| Intravenous, intramuscular | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Metabolism | Negligible |
| Half-life | 5.8–8.7 hours |
| Excretion | 33–67% renal, 35–45% biliary |
| Identifiers | |
|
73384-59-5 | |
| J01DD04 | |
| PubChem | CID 5479530 |
| DrugBank |
DB01212 |
| ChemSpider |
4586394 |
| UNII |
75J73V1629 |
| KEGG |
D07659 |
| ChEBI |
CHEBI:29007 |
| ChEMBL |
CHEMBL161 |
| Chemical data | |
| Formula | C18H18N8O7S3 |
| 554.58 g/mol | |
|
SMILES
| |
| |
| | |
Ceftriaxone (INN) /ˌsɛftraɪˈæksoʊn/ is an antibiotic useful for the treatment of a number of bacterial infections. It is a third-generation cephalosporin. Like other third-generation cephalosporins, it has broad-spectrum activity against Gram-positive bacteria and expanded Gram-negative coverage compared to second-generation agents. In most cases, it is considered to be equivalent to cefotaxime in terms of safety and efficacy. Ceftriaxone sodium is marketed by Hoffmann-La Roche under the trade name Rocephin.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[1]
Medical use
Ceftriaxone is used to treat a wide variety of serious infections caused by organisms that are resistant to most other antibiotics. It is often used (in combination, but not direct, with macrolide and/or aminoglycoside antibiotics) for the treatment of community-acquired or mild to moderate health care-associated pneumonia.
It is also a choice drug for treatment of bacterial meningitis caused by pneumococci, meningococci, Haemophilus influenzae, and susceptible enteric gram-negative rods, but not Listeria monocytogenes.[2]
Other uses included the treatment of acute bacterial otitis media, skin and skin structure infections, bone and joint infections, gonorrhea, intra-abdominal and urinary tract infections, pelvic inflammatory disease (PID), and bacterial septicemia. It also approved to be used in surgical (perioperative) prophylaxis.[3]
Pregnancy
Pregnancy category B. Ceftriaxone has not been observed to cause birth defects in animal studies, and preliminary studies in humans have shown no increase risk for birth defects.[4]
Breastfeeding
Low concentrations of ceftriaxone are excreted in breast milk. The manufacturer recommends that caution be exercised when administering ceftriaxone to women that breastfeed.[4]
Adverse effects
Ceftriaxone may precipitate in bile, causing biliary sludge, biliary pseudolithiasis, and gallstones, especially in children. It may cause allergic reactions similar to those caused by penicillin. Due to the 3-8% cross allergenicity with penicillins, caution should be used in those with a history of severe allergies to penicillin. Other common side effects include local irritation at the injections site, rash, and diarrhea. Hypoprothrombinaemia and bleeding are specific side-effects. Haemolysis is reported.[5][6][7] It has also been reported to cause post renal failure in children.[8]
Contraindications
Ceftriaxone should not be used in those with an allergy to ceftriaxone or any component of the formulation. It should not be used in hyperbilirubinemic neonates, particularly those who are premature because ceftriaxone is reported to displace bilirubin from albumin binding sites. It is contraindicated with concomitant use with intravenous calcium-containing solutions/products in neonates (≤28 days) [9] even if administered through different infusion lines due to rare fatal cases of calcium-ceftriaxone precipitations in neonatal lungs and kidneys.[10]
Spectrum of activity
Like other third-generation cephalosporins, ceftriaxone is active against citrobacter, S. marcescens, and beta-lactamase-producing strains of haemophilus and neisseria. However, unlike ceftazidime and cefoperazone, ceftriaxone does not have useful activity against Pseudomonas aeruginosa. It is not generally not active against enterobacter species, and its use should be avoided in the treatment of enterobacter infections even if the isolate appears susceptible because of the emergence of resistance. Like all other cephalosporins, it has no activity against enterococci, atypicals (Mycoplasma and Chlamydia), or Listeria.[2]
Mechanism of action
Ceftriaxone inhibits bacterial cell wall synthesis by means of binding to the penicillin-binding proteins (PBPs). Inhibition of PBPs would in turn inhibit the transpeptidation step in peptidoglycan synthesis which is required for bacterial cell walls. Like other cephalosporins, ceftriaxone is bacteriocidal and exhibits time-dependent killing.[11] (by inhibiting the cell wall synthesis).
Pharmacokinetics
The half-life is 7–8 hours. Like other third-generation cephalosporins, ceftriaxone penetrates body fluids and tissues well, and can achieve levels in the cerebrospinal fluid sufficient to inhibit most pathogens. The excretion is mainly through the biliary tract and no dose adjustment is required in renal impairment. With the exception cefoperazone, all other third-generation cephalosporins require dose adjustment in renal insufficiency. Ceftriaxone can be administered intravenously and intramuscularly. It is not available orally. To reduce the pain of intramuscular injection, ceftriaxone may be reconstituted with lidocaine.[12]
Chemistry
Ceftriaxone is a white crystalline powder readily soluble in water, sparingly soluble in methanol, and very slightly soluble in ethanol. The pH of a 1% aqueous solution is approximately 6.7.
The syn-configuration of the methoxyimino moiety confers resistance to β-lactamase enzymes produced by many Gram-negative bacteria. The stability of this configuration results in increased activity of ceftriaxone against otherwise-resistant Gram-negative bacteria. In place of the easily hydrolysed acetyl group of cefotaxime, ceftriaxone has a metabolically stable thiotriazinedione moiety.
Research
Ceftriaxone has also been investigated for efficacy in preventing relapse to cocaine addiction.[13]
Ceftriaxone seems to increase EAAT2 pump expression and activity[14] in the central nervous system and has therefore a potential to reduce glutamatergic toxicity.[15]
Ceftriaxone has been shown to have neuroprotective properties in a number of neurological disorders, including spinal muscular atrophy[16] and amyotrophic lateral sclerosis.[17] Despite earlier negative results in the 1990s, a large clinical trial was undertaken in 2006 to test ceftriaxone in ALS patients but was stopped early after it became clear that the results would not meet the pre-determined criteria for efficacy. [18]
References
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ 2.0 2.1 Katzung, Bertram (2009). Basic and Clinical Pharmacology, Eleventh Edition. New York: McGraw-Hill. pp. 783–784. ISBN 978-0-07-160405-5.
- ↑ Genetech USA. "Rochepin (ceftriaxone sodium) for injection" (PDF). Roche Group. Retrieved April 21, 2014.
- ↑ 4.0 4.1 "Ceftriaxone: Drug information". UpTpDate. Retrieved April 21, 2013.
- ↑ Shiffman ML, Keith FB, Moore EW (December 1990). "Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility". Gastroenterology 99 (6): 1772–8. PMID 2227290.
- ↑ Shrimali, JD; Patel, HV; Gumber, MR; Kute, VB; Shah, PR; Vanikar, AV; Trivedi, HL (Nov 2013). "Ceftriaxone induced immune hemolytic anemia with disseminated intravascular coagulation.". Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine 17 (6): 394–5. doi:10.4103/0972-5229.123465. PMC 3902580. PMID 24501497.
- ↑ Guleria, VS; Sharma, N; Amitabh, S; Nair, V (Sep–Oct 2013). "Ceftriaxone-induced hemolysis.". Indian journal of pharmacology 45 (5): 530–1. doi:10.4103/0253-7613.117758. PMC 3793531. PMID 24130395.
- ↑ Li, N.; Zhou, X.; Yuan, J.; Chen, G.; Jiang, H.; Zhang, W. (24 March 2014). "Ceftriaxone and Acute Renal Failure in Children". PEDIATRICS 133 (4): e917–e922. doi:10.1542/peds.2013-2103. PMID 24664092.
- ↑ "FDA Updates warning on Ceftriaxone-Calcium injection".
- ↑ Bradley JS; Wassel RT; Lee L; Nambiar S (April 2009). "Intravenous ceftriaxone and calcium in the neonate: assessing the risk for cardiopulmonary adverse events". Pediatrics 123 (4): e609–13. doi:10.1542/peds.2008-3080. PMID 19289450.
- ↑ Ceftriaxone: Drug information. Uptodate.com. Retrieved Oct 2013.
- ↑ Schichor A; Bernstein B; Weinerman H; Fitzgerald J; Yordan E; Schechter N (January 1994). "Lidocaine as a diluent for ceftriaxone in the treatment of gonorrhea. Does it reduce the pain of the injection?". Arch Pediatr Adolesc Med 148 (1): 72–5. doi:10.1001/archpedi.1994.02170010074017. PMID 8143016.
- ↑ Knackstedt LA; Melendez RI; Kalivas PW (August 2009). "Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine-seeking". Biol Psychiatry 67 (1): 81–4. doi:10.1016/j.biopsych.2009.07.018. PMC 2795043. PMID 19717140.
- ↑ Pharmacological evaluation of glutamate transporter 1 (GLT-1)-mediated neuroprotection following cerebral ischemia/reperfusion injury. Eur J Pharmacol. 2010 Jul 25;638(1-3):65-71. Epub 2010 Apr 24.
- ↑ Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB (2008). "Mechanism of Ceftriaxone Induction of Excitatory Amino Acid Transporter-2 Expression and Glutamate Uptake in Primary Human Astrocytes". The Journal of Biological Chemistry 283 (19): 13116–13123. doi:10.1074/jbc.M707697200. PMC 2442320. PMID 18326497.
- ↑ Hedlund, E. (2011). "The protective effects of beta-lactam antibiotics in motor neuron disorders". Experimental Neurology 231 (1): 14–18. doi:10.1016/j.expneurol.2011.06.002. PMID 21693120.
- ↑ Rothstein, J. D.; Patel, S.; Regan, M. R.; Haenggeli, C.; Huang, Y. H.; Bergles, D. E.; Jin, L.; Dykes Hoberg, M.; Vidensky, S.; Chung, D. S.; Toan, S. V.; Bruijn, L. I.; Su, Z. Z.; Gupta, P.; Fisher, P. B. (2005). "β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression". Nature 433 (7021): 73–77. doi:10.1038/nature03180. PMID 15635412.
- ↑ "Statement on the Clinical Trial of Ceftriaxone". The Northeast ALS Consortium (NEALS). 8 August 2012. Retrieved 10 May 2013.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||