Caspase-activated DNase
Caspase-Activated DNase (CAD) is a protein codified by a gene with 338 nucleotides located in a particular position on chromosome 1 (location 1p36.3). In addition, it breaks up the DNA during apoptosis and promotes the cell differentiation.
Despite this gene is present every cell, this protein is only expressed in different tissues and cell variety such as pancreas, heart, colon, leukocytes, prostate, ovary, placenta, kidney, spleen and thymus.[1]
It is also known as Caspase Activated Nuclease (CPAN), DNA Fragmentation Factor 40 (DFF-40), DFF2 and DFFB. Besides, there are other nomenclatures as a result of combining the previous ones.[1][2][3][4]
Structure
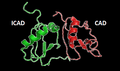
This heterodimer is an endonuclease [2][5][6] with a high content of cysteine residues.[4] It remains inactive in growing cells while it is associated with its inhibitor (ICAD, DNA fragmentation factor 45 kDa subunit, DFFA or DFF45) resulting into a complex ICAD-CAD.[1][2][4][5][7][8][9][10] Their dissociation allows DFF40 to oligomerize to form a large functional complex which is by itself an active DNase.[4][5][8][9][10]
DFF40 subunit or CAD
It weights 40 kDa. Moreover, it contains 3 domains making up a CAD monomer: C1 or N-terminal CAD; C2 which conform three separate α chains and, at last, C3 which is the largest and functionally the most important. What is more, combining C3’s amino acids leads to 5 α helices, 4 β lamina and a loop at the catalytic C-terminal which interact with each other. Therefore, a cavity (active site) where DNA can fit is produced, even though there is another binding region responsible for stable DNA complex during its fragmentation.[1][7][11]
DFF45 subunit or ICAD
DFFA is encoded by an alternatively encrypted mRNAs originating two distinct forms: short (ICAD-S) and long (ICAD-L), which act like a specific chaperone ensuring the correct CAD’s folding [3][4][10] Besides, it contains two aspartic acid residues (Asp117 and Asp224) where CAD is identified and, consequently, it stays bounded until Caspase-3 splist this union [3][7]
Activation process
Per usual in non-apoptotic growing cells caspase activated dnase is held in check inactivated in the cytoplasm thanks to the association with its inhibitor, inhibitor of caspase-activated DNase (ICAD) also known as DNA fragmentation factor 45 kDa (DFF45).
ICAD is encoded by alternatively spliced mRNAs which generate long (ICAD-L) and short (ICAD-S) forms of ICAD. Therefore ICAD has a double function; it acts as a CAD inhibitor and also as a chaperone for CAD synthesis assisting the correct assembly of the protein.[12]
ICAD has two caspase recognition sites at Asp117 and Asp224. CAD release from ICAD inhibition is achieved by cleavage of ICAD at these Asp residues by the caspase-3.[13]
Caspase-3 is activated in the apoptotic cell.[2] Caspase-3 activation is a cell requirement during early stages of the skeletal myoblast differentiation. Its catalytic site involves sulfohydryl group of Cys-285 and the imidazole ring of its His-237. The caspase-3 His-237 stabilizes the target Aspartate causing the break of the association of ICAD and CAD leaving the endonuclease CAD active allowing it to degrade chromosomal DNA.
Once the inhibitor is released and in order to properly function, two CAD monomers need to come together to form a functional dimer that has vertical symmetry.
Cell Differentiation
Caspase 3 is responsible for cellular differentiation, although it is unclear how this kind of protein can promote the cell apoptosis. Caspase signals resulting from the activation of nuclease CAD indicate that the cell differentiation is due to a CAD modification in chromatin structure.
CAD leads to the initiation of the DNA strand breakage, which occurs during terminal differentiation of some cell, such as skeletal muscle cell. Targeting of p21 promoter is responsible for inducing cell differentiation, which is promoted by modifying the DNA nuclear microenvironment. [14]
The cell diversity is originated by cell differentiation, which has been attributed to the activation of specific transcription factors. It also depends on the activity of a protein or a common signal. The factor that seems to induce more cell differentiation is caspase-3 protease. [15] This was identified as the penultimate stage of apoptosis pathways cell.
Some studies have shown that this differentiation is due to many CAD kinase substrates. Referring to the example of skeletal cells, their differentiation is associated to cleavage of the kinase MST1.[16]
Moreover, it has been seen that CAD participates in the formation of genome whose DNA breaks during early stages of the cell differentiation. Besides, Caspase 3 induces DNA breaks in the promoter of the factor p21 and this strand breakup is related to p21 gene expression.
Cell apoptotic death
The protein Caspase DNase is an endonuclease involved in the cell apoptotic process that facilitates the DNA breakup.[17] Cell apoptotic death is a process executed by cysteine proteases[18] that allows the animals to keep their homeostasis, also regulated by other mechanisms such as the growth and cell differentiation. This biological response is characterized by the chromosomal DNA’s degradation in tiny fragments within the nucleus of the cell.[19] After many investigations and research, it was possible to ensure that Caspase-activated DNase is the main responsible of this destruction due to a long list of stimuli.
One of the experiments carried out by the investigators in order to prove that theory was based on the introduction of mutated form of this protein inside both TF-1 human cells and Jurkat cells, which had already reacted to the usual (not mutated) form of the endonuclease and they had dead of apoptosis. As a result, these cells died taking into account this genetic modification but they did not show DNA breakup. This was the key evidence to prove that the CAD form is implicated in this part of the process because without its contribution the fragmentation did not take place.[20]
Later, it was found out that the way how this protein induces the DNA breakup is explained by its forms CAD and ICAD, which facilitate both the entry and exit in the nucleus of the cell.[19]
References
- ↑ 1.0 1.1 1.2 1.3 Davidson College. http://www.bio.davidson.edu/Immunology/Students/spring2006/Ryan/YFIP.html. Retrieved 16 October 2014. Missing or empty
|title=(help) - ↑ 2.0 2.1 2.2 2.3 Yuste, Victor J.; Sánchez-López, Isabel; Solé, Carme; Moubarak, Rana S.; Bayascas, José R.; Dolcet, Xavier; Encinas, Mario; Susin, Santos A.; Comella, Joan X. "The Contribution of Apoptosis-inducing Factor, Caspase-activated DNase, and Inhibitor of Caspase-activated DNase to the Nuclear Phenotype and DNA Degradation during Apoptosis". The Journal of Biological Chemistry. Retrieved 16 October 2014.
- ↑ 3.0 3.1 3.2 Hideki, Sakahira; Akihiro, Iwamatsu; Shigekazu, Nagata (2000). "Specific Chaperone-like Activity of Inhibitor of Caspase-activated DNase for Caspase-activated DNase". The Journal of Biological Chemistry 275 (11): 8091–8096. PMID 10713130.
- ↑ 4.0 4.1 4.2 4.3 4.4 Nagata, Shigekazu; Enari, Masato; Sakahira, Hideki (May 28, 1999). "Functional Differences of Two Forms of the Inhibitor of Caspase-activated DNase, ICAD-L, and ICAD-S". The Journal of Biological Chemistry 274 (22): 15740–15744. PMID 10336474. Retrieved 16 October 2014.
- ↑ 5.0 5.1 5.2 Neelakshi R.; Frisoni, Lorenza; Qin Shi; Marc, Monestier; Hernandez, Sairy; Craft, Joe; Luning Prak, Eline T.; Caricchio, Roberto (1 April 2013). "Caspase-Activated DNase is Required to Maintain Tolerance to Lupus Nuclear AutoAntigens". Arthritis Rheum 64 (4): 1247–1256. doi:10.1002/art.33448. PMC 3292632. PMID 22127758.
- ↑ Widlak, Piotr; Lanuszewska, Joanna; Cary, Robert B.; Garrard, William T. (May 14, 2003). "Subunit Structures and Stoichiometries of Human DNA Fragmentation Factor Proteins before and after Induction of Apoptosis". The Journal of Biological Chemistry 278 (29): 26915–26922. doi:10.1074/jbc.M303807200. PMID 12748178. Retrieved 20 October 2014.
- ↑ 7.0 7.1 7.2 Reh, Stefanie; Korn, Christian; Oleg, Gimadutdinow; Meiss, Gregor (October 19, 2005). "Structural Basis for Stable DNA Complex Formation by the Caspase-activated DNase". The Journal of Biological 280: 41707–41715. doi:10.1074/jbc.m509133200. Retrieved 16 October 2014.
- ↑ 8.0 8.1 Widlak, Piotr; Li, Peng; Wang, Xiaodong; Garrard, William T. (March 17, 2000). "Cleavage Preferences of the Apoptotic Endonuclease DFF40 (Caspase-activated DNase or Nuclease) on Naked DNA and Chromatin Substrates". The Journal of Biological Chemistry 275 (11): 8226–8232. doi:10.1074/jbc.275.11.8226. PMID 10713148. Retrieved 16 October 2014.
- ↑ 9.0 9.1 Sharif-Askar, Ehsan; Alam, Antoine; RheÂaume, Eric; J.Beresford, Paul; Scotto, Christian; Sharma, Kamal; Lee, Dennis; DeWolf, Walter E.; Nuttall, Mark E.; Lieberman, Judy; Sékaly, Rafick-Pierre (2001). "Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation". The EMBO Journal 20 (12): 3101–3113. doi:10.1093/emboj/20.12.3101. PMC 150191. PMID 11406587.
- ↑ 10.0 10.1 10.2 Liu, Xuesong; Zou, Hua; Widlak, Piotr; Garrard, William; Wang, Xiaodong (May 14, 1999). "Activation of the Apoptotic Endonuclease DFF40 (Caspase-activated DNase or Nuclease) OLIGOMERIZATION AND DIRECT INTERACTION WITH HISTONE H1". The Journal of Biological Chemistry 274 (20): 13836–13840. doi:10.1074/jbc.274.20.13836. PMID 10318789. Retrieved 16 October 2014.
- ↑ Uegaki, K; Otomo, T; Sakahira, H; Shimizu, M; Yumoto, N; Kyogoku, Y; Nagata, S; Yamazaki, T (14 April 2000). "Structure of the CAD domain of caspase-activated DNase and interaction with the CAD domain of its inhibitor". Journal of Molecular Biology 297 (5): 1121–8. doi:10.1006/jmbi.2000.3643. PMID 10764577. Retrieved 20 October 2014.
- ↑ http://www.rcsb.org/pdb/explore.do?structureId=1V0D#
- ↑ http://www.ncbi.nlm.nih.gov/gene/836
- ↑ D. Larsen, Brian; Rampalli, Shravanti; E. Burns, Leanne; Brunnete, Steve; Dilworth, F. Jeffrey; Megeney, Lynn A. (16 February 2010). "Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks". PMC. 107(9): 4230–4235 (9): 4230–4235. doi:10.1073/pnas.0913089107. PMC 2840077. PMID 20160104.
- ↑ Pasan, Fernando; Megerey, Lynn A (January 2007). "Is caspase-dependent apoptosis only cell differentiation taken to the extreme?". NCBI. 21(1):8-17 (1): 8–17. doi:10.1096/fj.06-5912hyp. PMID 17093139. Retrieved 16 October 2014.
- ↑ Kelly, John F.; Pasan, Fernando; Balazsi, Kim; Slack, Ruth S.; Manegey, Lynn A. (2 April 2002). "Caspase 3 activity is required for skeletal muscle differentiation". Science Sessions. vol. 99 no. 17 (17): 11025–11030. doi:10.1073/pnas.162172899. Retrieved 20 October 2014.
- ↑ Lai, S-K; Wong, C-H; Lee, Y-P; Li, H-I (10 December 2010). "Caspase-3-mediated degradation of condensin Cap-H regulates mitotic cell death". Cell Death & Differentiation 18 (6): 996–1004. doi:10.1038/cdd.2010.165. Retrieved 19 October 2014.
- ↑ S. Marsden, Vanessa; O'Connor, Liam; A. O'Reilly, Lorraine; Silke, John; Metcalf, Donald; G. Ekert, Paul; C. S. Huang, David; Cecconi, Francesco; Kuida, Keisuke; J. Tomaselli, Kevin; Roy, Sophie; W. Nicholson, Don; L. Vaux, David; Bouillet, Philippe; M. Adams, Jerry; Strasser, Andreas (10 October 2002). "Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome". Nature 419 (6907): 634–637. doi:10.1038/nature01101. Retrieved 17 October 2014.
- ↑ 19.0 19.1 Enari, Masato; Sakahira, Hideki; Yokohama, Hideki; Okawa, Katsuya; Iwamatsu, Akihiro; Nagata, Shigekazu (1 January 1998). "A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD". Nature 391 (6662): 43–50. doi:10.1038/34112. PMID 9422506. Retrieved 18 October 2014.
- ↑ McIlroy, Dorian; Sakahira, Hideki; Talanian, Robert; Nagata, Shigekazu (5 August 1999). "Involvement of caspase 3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli". Oncogene 18 (31): 4401–4408. doi:10.1038/sj.onc.1202868. PMID 10442630. Retrieved 16 October 2014.
Further Information
- Induction of Apoptosis (Video). Garland Science / YouTube. 2009. From Janeway's Immunobiology, 7th Edition, ISBN 978-0-8153-4123-9.