Carbon nanotube
| Part of a series of articles on |
| Nanomaterials |
|---|
| Fullerenes |
| Nanoparticles |
|
|
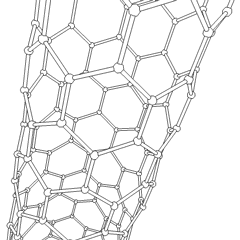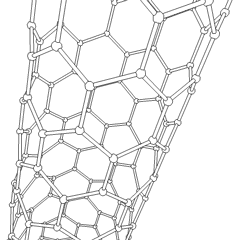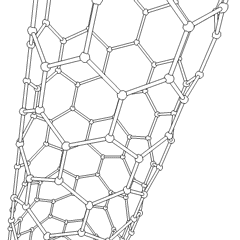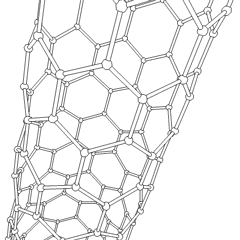
Carbon nanotubes (CNTs) are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1,[1] significantly larger than for any other material. These cylindrical carbon molecules have unusual properties, which are valuable for nanotechnology, electronics, optics and other fields of materials science and technology. In particular, owing to their extraordinary thermal conductivity and mechanical and electrical properties, carbon nanotubes find applications as additives to various structural materials. For instance, nanotubes form a tiny portion of the material(s) in some (primarily carbon fiber) baseball bats, golf clubs, car parts or damascus steel[2] [3]
Nanotubes are members of the fullerene structural family. Their name is derived from their long, hollow structure with the walls formed by one-atom-thick sheets of carbon, called graphene. These sheets are rolled at specific and discrete ("chiral") angles, and the combination of the rolling angle and radius decides the nanotube properties; for example, whether the individual nanotube shell is a metal or semiconductor. Nanotubes are categorized as single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs). Individual nanotubes naturally align themselves into "ropes" held together by van der Waals forces, more specifically, pi-stacking.
Applied quantum chemistry, specifically, orbital hybridization best describes chemical bonding in nanotubes. The chemical bonding of nanotubes is composed entirely of sp2 bonds, similar to those of graphite. These bonds, which are stronger than the sp3 bonds found in alkanes and diamond, provide nanotubes with their unique strength.
Types of carbon nanotubes and related structures
Terminology
There is no consensus on some terms describing carbon nanotubes in scientific literature: both "-wall" and "-walled" are being used in combination with "single", "double", "triple" or "multi", and the letter C is often omitted in the abbreviation; for example, multi-walled carbon nanotube (MWNT).
Single-walled
-
Armchair (n,n) i.e.: m=n
-
The translation vector is bent, while the chiral vector stays straight
-
Graphene nanoribbon
-
The chiral vector is bent, while the translation vector stays straight
-
Zigzag (n,0)
-
Chiral (n,m)
-
n and m can be counted at the end of the tube
-
Graphene nanoribbon



Most single-walled nanotubes (SWNTs) have a diameter of close to 1 nanometer, with a tube length that can be many millions of times longer. The structure of a SWNT can be conceptualized by wrapping a one-atom-thick layer of graphite called graphene into a seamless cylinder. The way the graphene sheet is wrapped is represented by a pair of indices (n,m). The integers n and m denote the number of unit vectors along two directions in the honeycomb crystal lattice of graphene. If m = 0, the nanotubes are called zigzag nanotubes, and if n = m, the nanotubes are called armchair nanotubes. Otherwise, they are called chiral. The diameter of an ideal nanotube can be calculated from its (n,m) indices as follows
where a = 0.246 nm.
SWNTs are an important variety of carbon nanotube because most of their properties change significantly with the (n,m) values, and this dependence is non-monotonic (see Kataura plot). In particular, their band gap can vary from zero to about 2 eV and their electrical conductivity can show metallic or semiconducting behavior. Single-walled nanotubes are likely candidates for miniaturizing electronics. The most basic building block of these systems is the electric wire, and SWNTs with diameters of an order of a nanometer can be excellent conductors.[4][5] One useful application of SWNTs is in the development of the first intermolecular field-effect transistors (FET). The first intermolecular logic gate using SWCNT FETs was made in 2001.[6] A logic gate requires both a p-FET and an n-FET. Because SWNTs are p-FETs when exposed to oxygen and n-FETs otherwise, it is possible to protect half of an SWNT from oxygen exposure, while exposing the other half to oxygen. This results in a single SWNT that acts as a not logic gate with both p and n-type FETs within the same molecule.
.jpg)
Single-walled nanotubes are dropping precipitously in price, from around $1500 per gram as of 2000 to retail prices of around $50 per gram of as-produced 40–60% by weight SWNTs as of March 2010.
SWNTs have been viewed as too expensive for widespread application but are forecast to make a large impact in electronics applications by 2020 according to The Global Market for Carbon Nanotubes report.
Multi-walled

Multi-walled nanotubes (MWNTs) consist of multiple rolled layers (concentric tubes) of graphene. There are two models that can be used to describe the structures of multi-walled nanotubes. In the Russian Doll model, sheets of graphite are arranged in concentric cylinders, e.g., a (0,8) single-walled nanotube (SWNT) within a larger (0,17) single-walled nanotube. In the Parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled newspaper. The interlayer distance in multi-walled nanotubes is close to the distance between graphene layers in graphite, approximately 3.4 Å. The Russian Doll structure is observed more commonly. Its individual shells can be described as SWNTs, which can be metallic or semiconducting. Because of statistical probability and restrictions on the relative diameters of the individual tubes, one of the shells, and thus the whole MWNT, is usually a zero-gap metal.
Double-walled carbon nanotubes (DWNTs) form a special class of nanotubes because their morphology and properties are similar to those of SWNTs but their resistance to chemicals is significantly improved. This is especially important when functionalization is required (this means grafting of chemical functions at the surface of the nanotubes) to add new properties to the CNT. In the case of SWNTs, covalent functionalization will break some C=C double bonds, leaving "holes" in the structure on the nanotube and, thus, modifying both its mechanical and electrical properties. In the case of DWNTs, only the outer wall is modified. DWNT synthesis on the gram-scale was first proposed in 2003[7] by the CCVD technique, from the selective reduction of oxide solutions in methane and hydrogen.
The telescopic motion ability of inner shells[8] and their unique mechanical properties[9] will permit the use of multi-walled nanotubes as main movable arms in coming nanomechanical devices. Retraction force that occurs to telescopic motion caused by the Lennard-Jones interaction between shells and its value is about 1.5 nN.[10]
Torus
In theory, a nanotorus is a carbon nanotube bent into a torus (doughnut shape). Nanotori are predicted to have many unique properties, such as magnetic moments 1000 times larger than previously expected for certain specific radii.[11] Properties such as magnetic moment, thermal stability, etc. vary widely depending on radius of the torus and radius of the tube.[11][12]
Nanobud
Carbon nanobuds are a newly created material combining two previously discovered allotropes of carbon: carbon nanotubes and fullerenes. In this new material, fullerene-like "buds" are covalently bonded to the outer sidewalls of the underlying carbon nanotube. This hybrid material has useful properties of both fullerenes and carbon nanotubes. In particular, they have been found to be exceptionally good field emitters. In composite materials, the attached fullerene molecules may function as molecular anchors preventing slipping of the nanotubes, thus improving the composite’s mechanical properties.
Three-dimensional carbon nanotube architectures
Recently, several studies have highlighted the prospect of using carbon nanotubes as building blocks to fabricate three-dimensional macroscopic (>1mm in all three dimensions) all-carbon devices. Lalwani et al. have reported a novel radical initiated thermal crosslinking method to fabricate macroscopic, free-standing, porous, all-carbon scaffolds using single- and multi-walled carbon nanotubes as building blocks.[13] These scaffolds possess macro-, micro-, and nano- structured pores and the porosity can be tailored for specific applications. These 3D all-carbon scaffolds/architectures may be used for the fabrication of the next generation of energy storage, supercapacitors, field emission transistors, high-performance catalysis, photovoltaics, and biomedical devices and implants. In addition, the mechanical behaviour of carbon nanotube micro-architectures can easily be modified by the infiltration and deposition of thin conformal coatings.[14]
Graphenated carbon nanotubes (g-CNTs)

Graphenated CNTs are a relatively new hybrid that combines graphitic foliates grown along the sidewalls of multiwalled or bamboo style CNTs. Yu et al.[15] reported on "chemically bonded graphene leaves" growing along the sidewalls of CNTs. Stoner et al.[16] described these structures as "graphenated CNTs" and reported in their use for enhanced supercapacitor performance. Hsu et al. further reported on similar structures formed on carbon fiber paper, also for use in supercapacitor applications.[17] The foliate density can vary as a function of deposition conditions (e.g. temperature and time) with their structure ranging from few layers of graphene (< 10) to thicker, more graphite-like.[18]
The fundamental advantage of an integrated graphene-CNT structure is the high surface area three-dimensional framework of the CNTs coupled with the high edge density of graphene. Graphene edges provide significantly higher charge density and reactivity than the basal plane, but they are difficult to arrange in a three-dimensional, high volume-density geometry. CNTs are readily aligned in a high density geometry (i.e., a vertically aligned forest)[19] but lack high charge density surfaces—the sidewalls of the CNTs are similar to the basal plane of graphene and exhibit low charge density except where edge defects exist. Depositing a high density of graphene foliates along the length of aligned CNTs can significantly increase the total charge capacity per unit of nominal area as compared to other carbon nanostructures.[20]
Nitrogen-doped carbon nanotubes
Nitrogen doped carbon nanotubes (N-CNTs) can be produced through five main methods, chemical vapor deposition,[21][22] high-temperature and high-pressure reactions, gas-solid reaction of amorphous carbon with NH3 at high temperature,[23] solid reaction,[24] and solvothermal synthesis.[25]
N-CNTs can also be prepared by a CVD method of pyrolyzing melamine under Ar at elevated temperatures of 800–980 °C. However synthesis by CVD of melamine results in the formation of bamboo-structured CNTs. XPS spectra of grown N-CNTs reveal nitrogen in five main components, pyridinic nitrogen, pyrrolic nitrogen, quaternary nitrogen, and nitrogen oxides. Furthermore synthesis temperature affects the type of nitrogen configuration.[22]
Nitrogen doping plays a pivotal role in lithium storage, as it creates defects in the CNT walls allowing for Li ions to diffuse into interwall space. It also increases capacity by providing more favorable bind of N-doped sites. N-CNTs are also much more reactive to metal oxide nanoparticle deposition which can further enhance storage capacity, especially in anode materials for Li-ion batteries.[26] However boron-doped nanotubes have been shown to make batteries with triple capacity.[27]
Peapod
A carbon peapod[28][29] is a novel hybrid carbon material which traps fullerene inside a carbon nanotube. It can possess interesting magnetic properties with heating and irradiation. It can also be applied as an oscillator during theoretical investigations and predictions.[30][31]
Cup-stacked carbon nanotubes
Cup-stacked carbon nanotubes (CSCNTs) differ from other quasi-1D carbon structures, which normally behave as quasi-metallic conductors of electrons. CSCNTs exhibit semiconducting behaviors due to the stacking microstructure of graphene layers.[32]
Extreme carbon nanotubes
The observation of the longest carbon nanotubes grown so far are over 1/2 m (550 mm long) was reported in 2013.[33] These nanotubes were grown on Si substrates using an improved chemical vapor deposition (CVD) method and represent electrically uniform arrays of single-walled carbon nanotubes.[1]
The shortest carbon nanotube is the organic compound cycloparaphenylene, which was synthesized in early 2009.[34][35]
The thinnest carbon nanotube is the armchair (2,2) CNT with a diameter of 3 Å. This nanotube was grown inside a multi-walled carbon nanotube. Assigning of carbon nanotube type was done by a combination of high-resolution transmission electron microscopy (HRTEM), Raman spectroscopy and density functional theory (DFT) calculations.[36]
The thinnest freestanding single-walled carbon nanotube is about 4.3 Å in diameter. Researchers suggested that it can be either (5,1) or (4,2) SWCNT, but the exact type of carbon nanotube remains questionable.[37] (3,3), (4,3) and (5,1) carbon nanotubes (all about 4 Å in diameter) were unambiguously identified using aberration-corrected high-resolution transmission electron microscopy inside double-walled CNTs.[38]
The highest density of CNTs was achieved in 2013, grown on a conductive titanium-coated copper surface that was coated with co-catalysts cobalt and molybdenum at lower than typical temperatures of 450 °C. The tubes averaged a height of 0.38 μm and a mass density of 1.6 g cm−3. The material showed ohmic conductivity (lowest resistance ∼22 kΩ).[39][40]
Properties
Strength
Carbon nanotubes are the strongest and stiffest materials yet discovered in terms of tensile strength and elastic modulus respectively. This strength results from the covalent sp2 bonds formed between the individual carbon atoms. In 2000, a multi-walled carbon nanotube was tested to have a tensile strength of 63 gigapascals (9,100,000 psi).[41] (For illustration, this translates into the ability to endure tension of a weight equivalent to 6,422 kilograms-force (62,980 N; 14,160 lbf) on a cable with cross-section of 1 square millimetre (0.0016 sq in).) Further studies, such as one conducted in 2008, revealed that individual CNT shells have strengths of up to ~100 gigapascals (15,000,000 psi), which is in agreement with quantum/atomistic models.[42] Since carbon nanotubes have a low density for a solid of 1.3 to 1.4 g/cm3,[43] its specific strength of up to 48,000 kN·m·kg−1 is the best of known materials, compared to high-carbon steel's 154 kN·m·kg−1.
Under excessive tensile strain, the tubes will undergo plastic deformation, which means the deformation is permanent. This deformation begins at strains of approximately 5% and can increase the maximum strain the tubes undergo before fracture by releasing strain energy.
Although the strength of individual CNT shells is extremely high, weak shear interactions between adjacent shells and tubes lead to significant reduction in the effective strength of multi-walled carbon nanotubes and carbon nanotube bundles down to only a few GPa.[44] This limitation has been recently addressed by applying high-energy electron irradiation, which crosslinks inner shells and tubes, and effectively increases the strength of these materials to ~60 GPa for multi-walled carbon nanotubes[42] and ~17 GPa for double-walled carbon nanotube bundles.[44]
CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional, or bending stress.[45]
| Material | Young's modulus (TPa) | Tensile strength (GPa) | Elongation at break (%) |
|---|---|---|---|
| SWNTE | ~1 (from 1 to 5) | 13–53 | 16 |
| Armchair SWNTT | 0.94 | 126.2 | 23.1 |
| Zigzag SWNTT | 0.94 | 94.5 | 15.6–17.5 |
| Chiral SWNT | 0.92 | ||
| MWNTE | 0.2[41]–0.8[50]–0.95[41] | 11[41]–63[41]–150[50] | |
| Stainless steelE | 0.186[51]–0.214[52] | 0.38[51]–1.55[52] | 15–50 |
| Kevlar–29&149E | 0.06–0.18[53] | 3.6–3.8[53] | ~2 |
EExperimental observation; TTheoretical prediction
The above discussion referred to axial properties of the nanotube, whereas simple geometrical considerations suggest that carbon nanotubes should be much softer in the radial direction than along the tube axis. Indeed, TEM observation of radial elasticity suggested that even the van der Waals forces can deform two adjacent nanotubes.[54] Nanoindentation experiments, performed by several groups on multiwalled carbon nanotubes[55][56] and tapping/contact mode atomic force microscope measurements performed on single-walled carbon nanotubes,[57] indicated a Young's modulus of the order of several GPa, confirming that CNTs are indeed rather soft in the radial direction.
Hardness
Standard single-walled carbon nanotubes can withstand a pressure up to 25 GPa without deformation. They then undergo a transformation to superhard phase nanotubes. Maximum pressures measured using current experimental techniques are around 55 GPa. However, these new superhard phase nanotubes collapse at an even higher, albeit unknown, pressure.
The bulk modulus of superhard phase nanotubes is 462 to 546 GPa, even higher than that of diamond (420 GPa for single diamond crystal).[58]
Kinetic properties
Multi-walled nanotubes are multiple concentric nanotubes precisely nested within one another. These exhibit a striking telescoping property whereby an inner nanotube core may slide, almost without friction, within its outer nanotube shell, thus creating an atomically perfect linear or rotational bearing. This is one of the first true examples of molecular nanotechnology, the precise positioning of atoms to create useful machines. Already, this property has been utilized to create the world's smallest rotational motor.[59] Future applications such as a gigahertz mechanical oscillator are also envisioned.
Electrical properties

Because of the symmetry and unique electronic structure of graphene, the structure of a nanotube strongly affects its electrical properties. For a given (n,m) nanotube, if n = m, the nanotube is metallic; if n − m is a multiple of 3, then the nanotube is semiconducting with a very small band gap, otherwise the nanotube is a moderate semiconductor. Thus all armchair (n = m) nanotubes are metallic, and nanotubes (6,4), (9,1), etc. are semiconducting.[60]
However, this rule has exceptions, because curvature effects in small diameter tubes can strongly influence electrical properties. Thus, a (5,0) SWCNT that should be semiconducting in fact is metallic according to the calculations. Likewise, zigzag and chiral SWCNTs with small diameters that should be metallic have a finite gap (armchair nanotubes remain metallic).[60] In theory, metallic nanotubes can carry an electric current density of 4 × 109 A/cm2, which is more than 1,000 times greater than those of metals such as copper,[61] where for copper interconnects current densities are limited by electromigration.
Because of its nanoscale cross-section, electrons propagate only along the tube's axis. As a result, carbon nanotubes are frequently referred to as one-dimensional conductors. The maximum electrical conductance of a single-walled carbon nanotube is 2G0, where G0 = 2e2/h is the conductance of a single ballistic quantum channel.[62]
Intrinsic superconductivity has been reported,[63] although other experiments found no evidence of this, leaving the claim a subject of debate.[64]
Optical properties
Thermal properties
All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as "ballistic conduction", but good insulators laterally to the tube axis. Measurements show that a SWNT has a room-temperature thermal conductivity along its axis of about 3500 W·m−1·K−1;[65] compare this to copper, a metal well known for its good thermal conductivity, which transmits 385 W·m−1·K−1. A SWNT has a room-temperature thermal conductivity across its axis (in the radial direction) of about 1.52 W·m−1·K−1,[66] which is about as thermally conductive as soil. The temperature stability of carbon nanotubes is estimated to be up to 2800 °C in vacuum and about 750 °C in air.[67]
Defects
As with any material, the existence of a crystallographic defect affects the material properties. Defects can occur in the form of atomic vacancies. High levels of such defects can lower the tensile strength by up to 85%. An important example is the Stone Wales defect, which creates a pentagon and heptagon pair by rearrangement of the bonds. Because of the very small structure of CNTs, the tensile strength of the tube is dependent on its weakest segment in a similar manner to a chain, where the strength of the weakest link becomes the maximum strength of the chain.
Crystallographic defects also affect the tube's electrical properties. A common result is lowered conductivity through the defective region of the tube. A defect in armchair-type tubes (which can conduct electricity) can cause the surrounding region to become semiconducting, and single monatomic vacancies induce magnetic properties.[68]
Crystallographic defects strongly affect the tube's thermal properties. Such defects lead to phonon scattering, which in turn increases the relaxation rate of the phonons. This reduces the mean free path and reduces the thermal conductivity of nanotube structures. Phonon transport simulations indicate that substitutional defects such as nitrogen or boron will primarily lead to scattering of high-frequency optical phonons. However, larger-scale defects such as Stone Wales defects cause phonon scattering over a wide range of frequencies, leading to a greater reduction in thermal conductivity.[69]
Toxicity
The toxicity of carbon nanotubes has been an important question in nanotechnology. As of 2007, such research has just begun. The data are still fragmentary and subject to criticism. Preliminary results highlight the difficulties in evaluating the toxicity of this heterogeneous material. Parameters such as structure, size distribution, surface area, surface chemistry, surface charge, and agglomeration state as well as purity of the samples, have considerable impact on the reactivity of carbon nanotubes. However, available data clearly show that, under some conditions, nanotubes can cross membrane barriers, which suggests that, if raw materials reach the organs, they can induce harmful effects such as inflammatory and fibrotic reactions.[70][71]
Under certain conditions CNTs can enter human cells and accumulate in the cytoplasm, causing cell death.[72]
Results of rodent studies collectively show that regardless of the process by which CNTs were synthesized and the types and amounts of metals they contained, CNTs were capable of producing inflammation, epithelioid granulomas (microscopic nodules), fibrosis, and biochemical/toxicological changes in the lungs.[73] Comparative toxicity studies in which mice were given equal weights of test materials showed that SWCNTs were more toxic than quartz, which is considered a serious occupational health hazard when chronically inhaled. As a control, ultrafine carbon black was shown to produce minimal lung responses.[74]
Carbon nanotubes deposit in the alveolar ducts by aligning lengthwise with the airways; the nanotubes will often combine with metals.[75] The needle-like fiber shape of CNTs is similar to asbestos fibers. This raises the idea that widespread use of carbon nanotubes may lead to pleural mesothelioma, a cancer of the lining of the lungs, or peritoneal mesothelioma, a cancer of the lining of the abdomen (both caused by exposure to asbestos). A recently published pilot study supports this prediction.[76] Scientists exposed the mesothelial lining of the body cavity of mice to long multiwalled carbon nanotubes and observed asbestos-like, length-dependent, pathogenic behavior that included inflammation and formation of lesions known as granulomas. Authors of the study conclude:
This is of considerable importance, because research and business communities continue to invest heavily in carbon nanotubes for a wide range of products under the assumption that they are no more hazardous than graphite. Our results suggest the need for further research and great caution before introducing such products into the market if long-term harm is to be avoided.[76]
Although further research is required, the available data suggest that under certain conditions, especially those involving chronic exposure, carbon nanotubes can pose a serious risk to human health.[70][72][74][76]
Synthesis

Techniques have been developed to produce nanotubes in sizable quantities, including arc discharge, laser ablation, high-pressure carbon monoxide disproportionation, and chemical vapor deposition (CVD). Most of these processes take place in a vacuum or with process gases. CVD growth of CNTs can occur in vacuum or at atmospheric pressure. Large quantities of nanotubes can be synthesized by these methods; advances in catalysis and continuous growth are making CNTs more commercially viable.
Arc discharge
Nanotubes were observed in 1991 in the carbon soot of graphite electrodes during an arc discharge, by using a current of 100 amps, that was intended to produce fullerenes.[77] However the first macroscopic production of carbon nanotubes was made in 1992 by two researchers at NEC's Fundamental Research Laboratory.[78] The method used was the same as in 1991. During this process, the carbon contained in the negative electrode sublimates because of the high-discharge temperatures.
The yield for this method is up to 30% by weight and it produces both single- and multi-walled nanotubes with lengths of up to 50 micrometers with few structural defects.[43]
Laser ablation
In laser ablation, a pulsed laser vaporizes a graphite target in a high-temperature reactor while an inert gas is bled into the chamber. Nanotubes develop on the cooler surfaces of the reactor as the vaporized carbon condenses. A water-cooled surface may be included in the system to collect the nanotubes.
This process was developed by Dr. Richard Smalley and co-workers at Rice University, who at the time of the discovery of carbon nanotubes, were blasting metals with a laser to produce various metal molecules. When they heard of the existence of nanotubes they replaced the metals with graphite to create multi-walled carbon nanotubes.[79] Later that year the team used a composite of graphite and metal catalyst particles (the best yield was from a cobalt and nickel mixture) to synthesize single-walled carbon nanotubes.[80]
The laser ablation method yields around 70% and produces primarily single-walled carbon nanotubes with a controllable diameter determined by the reaction temperature. However, it is more expensive than either arc discharge or chemical vapor deposition.[43]
Plasma torch
Single-walled carbon nanotubes can also be synthesized by a thermal plasma method. It was first invented in 2000 at INRS (Institut National de la Recherche Scientifique in Varennes, Canada), by Olivier Smiljanic. In this method, the aim is to reproduce the conditions prevailing in the arc discharge and laser ablation approaches, but a carbon-containing gas is used instead of graphite vapors to supply the carbon necessary for the production of SWNT. Doing so, the growth of SWNT is more efficient (decomposing a carbon containing gas can be 10 times less energy-consuming than graphite vaporization). It is also continuous and occurs at low cost. To produce a continuous process, a gas mixture composed of argon, ethylene and ferrocene is introduced into a microwave plasma torch, where it is atomized by the atmospheric pressure plasma, which has the form of an intense 'flame'. The fumes created by the flame are found to contain SWNT, metallic and carbon nanoparticles and amorphous carbon.[81][82]
Another way to produce single-walled carbon nanotubes with a plasma torch, is to use the induction thermal plasma method, implemented in 2005 by groups from the University of Sherbrooke and the National Research Council of Canada.[83] The method is similar to arc-discharge in that both use ionized gas to reach the high temperature necessary to vaporize carbon-containing substances and the metal catalysts necessary for the ensuing nanotube growth. The thermal plasma is induced by high frequency oscillating currents in a coil, and is maintained in flowing inert gas. Typically, a feedstock of carbon black and metal catalyst particles is fed into the plasma, and then cooled down to form single-walled carbon nanotubes. Different single-wall carbon nanotube diameter distributions can be synthesized.
The induction thermal plasma method can produce up to 2 grams of nanotube material per minute, which is higher than the arc-discharge or the laser ablation methods.
Chemical vapor deposition (CVD)
The catalytic vapor phase deposition of carbon was reported in 1952[84] and 1959,[85] but it was not until 1993[86] that carbon nanotubes were formed by this process. In 2007, researchers at the University of Cincinnati (UC) developed a process to grow aligned carbon nanotube arrays of length 18 mm on a FirstNano ET3000 carbon nanotube growth system.[87]
During CVD, a substrate is prepared with a layer of metal catalyst particles, most commonly nickel, cobalt,[88] iron, or a combination.[89] The metal nanoparticles can also be produced by other ways, including reduction of oxides or oxides solid solutions. The diameters of the nanotubes that are to be grown are related to the size of the metal particles. This can be controlled by patterned (or masked) deposition of the metal, annealing, or by plasma etching of a metal layer. The substrate is heated to approximately 700 °C. To initiate the growth of nanotubes, two gases are bled into the reactor: a process gas (such as ammonia, nitrogen or hydrogen) and a carbon-containing gas (such as acetylene, ethylene, ethanol or methane). Nanotubes grow at the sites of the metal catalyst; the carbon-containing gas is broken apart at the surface of the catalyst particle, and the carbon is transported to the edges of the particle, where it forms the nanotubes. This mechanism is still being studied.[90] The catalyst particles can stay at the tips of the growing nanotube during growth, or remain at the nanotube base, depending on the adhesion between the catalyst particle and the substrate.[91] Thermal catalytic decomposition of hydrocarbon has become an active area of research and can be a promising route for the bulk production of CNTs. Fluidised bed reactor is the most widely used reactor for CNT preparation. Scale-up of the reactor is the major challenge.[92][93]
CVD is the most widely used method for the production of carbon nanotubes.[94] For this purpose, the metal nanoparticles are mixed with a catalyst support such as MgO or Al2O3 to increase the surface area for higher yield of the catalytic reaction of the carbon feedstock with the metal particles. One issue in this synthesis route is the removal of the catalyst support via an acid treatment, which sometimes could destroy the original structure of the carbon nanotubes. However, alternative catalyst supports that are soluble in water have proven effective for nanotube growth.[95]
If a plasma is generated by the application of a strong electric field during growth (plasma-enhanced chemical vapor deposition), then the nanotube growth will follow the direction of the electric field.[96] By adjusting the geometry of the reactor it is possible to synthesize vertically aligned carbon nanotubes[97] (i.e., perpendicular to the substrate), a morphology that has been of interest to researchers interested in electron emission from nanotubes. Without the plasma, the resulting nanotubes are often randomly oriented. Under certain reaction conditions, even in the absence of a plasma, closely spaced nanotubes will maintain a vertical growth direction resulting in a dense array of tubes resembling a carpet or forest.
Of the various means for nanotube synthesis, CVD shows the most promise for industrial-scale deposition, because of its price/unit ratio, and because CVD is capable of growing nanotubes directly on a desired substrate, whereas the nanotubes must be collected in the other growth techniques. The growth sites are controllable by careful deposition of the catalyst.[98] In 2007, a team from Meijo University demonstrated a high-efficiency CVD technique for growing carbon nanotubes from camphor.[99] Researchers at Rice University, until recently led by the late Richard Smalley, have concentrated upon finding methods to produce large, pure amounts of particular types of nanotubes. Their approach grows long fibers from many small seeds cut from a single nanotube; all of the resulting fibers were found to be of the same diameter as the original nanotube and are expected to be of the same type as the original nanotube.[100]
Super-growth CVD
Super-growth CVD (water-assisted chemical vapor deposition) was developed by Kenji Hata, Sumio Iijima and co-workers at AIST, Japan.[101] In this process, the activity and lifetime of the catalyst are enhanced by addition of water into the CVD reactor. Dense millimeter-tall nanotube "forests", aligned normal to the substrate, were produced. The forests height could be expressed, as
In this equation, β is the initial growth rate and  is the characteristic catalyst lifetime.[102]
is the characteristic catalyst lifetime.[102]
Their specific surface exceeds 1,000 m2/g (capped) or 2,200 m2/g (uncapped),[103] surpassing the value of 400–1,000 m2/g for HiPco samples. The synthesis efficiency is about 100 times higher than for the laser ablation method. The time required to make SWNT forests of the height of 2.5 mm by this method was 10 minutes in 2004. Those SWNT forests can be easily separated from the catalyst, yielding clean SWNT material (purity >99.98%) without further purification. For comparison, the as-grown HiPco CNTs contain about 5–35%[104] of metal impurities; it is therefore purified through dispersion and centrifugation that damages the nanotubes. Super-growth avoids this problem. Patterned highly organized single-walled nanotube structures were successfully fabricated using the super-growth technique.
The mass density of super-growth CNTs is about 0.037 g/cm3.[105][106] It is much lower than that of conventional CNT powders (~1.34 g/cm3), probably because the latter contain metals and amorphous carbon.
The super-growth method is basically a variation of CVD. Therefore, it is possible to grow material containing SWNT, DWNTs and MWNTs, and to alter their ratios by tuning the growth conditions.[107] Their ratios change by the thinness of the catalyst. Many MWNTs are included so that the diameter of the tube is wide.[106]
The vertically aligned nanotube forests originate from a "zipping effect" when they are immersed in a solvent and dried. The zipping effect is caused by the surface tension of the solvent and the van der Waals forces between the carbon nanotubes. It aligns the nanotubes into a dense material, which can be formed in various shapes, such as sheets and bars, by applying weak compression during the process. Densification increases the Vickers hardness by about 70 times and density is 0.55 g/cm3. The packed carbon nanotubes are more than 1 mm long and have a carbon purity of 99.9% or higher; they also retain the desirable alignment properties of the nanotubes forest.[108]
Natural, incidental, and controlled flame environments
Fullerenes and carbon nanotubes are not necessarily products of high-tech laboratories; they are commonly formed in such mundane places as ordinary flames,[109] produced by burning methane,[110] ethylene,[111] and benzene,[112] and they have been found in soot from both indoor and outdoor air.[113] However, these naturally occurring varieties can be highly irregular in size and quality because the environment in which they are produced is often highly uncontrolled. Thus, although they can be used in some applications, they can lack in the high degree of uniformity necessary to satisfy the many needs of both research and industry. Recent efforts have focused on producing more uniform carbon nanotubes in controlled flame environments.[114][115][116][117] Such methods have promise for large-scale, low-cost nanotube synthesis based on theoretical models,[118] though they must compete with rapidly developing large scale CVD production.
Removal of catalysts
Nanoscale metal catalysts are important ingredients for fixed- and fluidized-bed CVD synthesis of CNTs. They allow increasing the growth efficiency of CNTs and may give control over their structure and chirality.[119] During synthesis, catalysts can convert carbon precursors into tubular carbon structures but can also form encapsulating carbon overcoats. Together with metal oxide supports they may therefore attach to or become incorporated into the CNT product.[120] The presence of metal impurities can be problematic for many applications. Especially catalyst metals like nickel, cobalt or yttrium may be of toxicological concern.[121] While unencapsulated catalyst metals may be readily removable by acid washing, encapsulated ones require oxidative treatment for opening their carbon shell.[122] The effective removal of catalysts, especially of encapsulated ones, while preserving the CNT structure is a challenge and has been addressed in many studies.[123][124] A new approach to break carbonaceaous catalyst encapsulations is based on rapid thermal annealing.[125]
Application-related issues

Many electronic applications of carbon nanotubes crucially rely on techniques of selectively producing either semiconducting or metallic CNTs, preferably of a certain chirality. Several methods of separating semiconducting and metallic CNTs are known, but most of them are not yet suitable for large-scale technological processes. The most efficient method relies on density-gradient ultracentrifugation, which separates surfactant-wrapped nanotubes by the minute difference in their density. This density difference often translates into difference in the nanotube diameter and (semi)conducting properties.[126] Another method of separation uses a sequence of freezing, thawing, and compression of SWNTs embedded in agarose gel. This process results in a solution containing 70% metallic SWNTs and leaves a gel containing 95% semiconducting SWNTs. The diluted solutions separated by this method show various colors.[127][128] The separated carbon nanotubes using this method have been applied to electrodes, e.g. electric double-layer capacitor.[129] Moreover, SWNTs can be separated by the column chromatography method. Yield is 95% in semiconductor type SWNT and 90% in metallic type SWNT.[130]
In addition to separation of semiconducting and metallic SWNTs, it is possible to sort SWNTs by length, diameter, and chirality. The highest resolution length sorting, with length variation of <10%, has thus far been achieved by size exclusion chromatography (SEC) of DNA-dispersed carbon nanotubes (DNA-SWNT).[131] SWNT diameter separation has been achieved by density-gradient ultracentrifugation (DGU)[132] using surfactant-dispersed SWNTs and by ion-exchange chromatography (IEC) for DNA-SWNT.[133] Purification of individual chiralities has also been demonstrated with IEC of DNA-SWNT: specific short DNA oligomers can be used to isolate individual SWNT chiralities. Thus far, 12 chiralities have been isolated at purities ranging from 70% for (8,3) and (9,5) SWNTs to 90% for (6,5), (7,5) and (10,5)SWNTs.[134] There have been successful efforts to integrate these purified nanotubes into devices, e. g. FETs.[135]
An alternative to separation is development of a selective growth of semiconducting or metallic CNTs. Recently, a new CVD recipe that involves a combination of ethanol and methanol gases and quartz substrates resulting in horizontally aligned arrays of 95–98% semiconducting nanotubes was announced.[136]
Nanotubes are usually grown on nanoparticles of magnetic metal (Fe, Co), which facilitates production of electronic (spintronic) devices. In particular, control of current through a field-effect transistor by magnetic field has been demonstrated in such a single-tube nanostructure.[137]
Current applications
Current use and application of nanotubes has mostly been limited to the use of bulk nanotubes, which is a mass of rather unorganized fragments of nanotubes. Bulk nanotube materials may never achieve a tensile strength similar to that of individual tubes, but such composites may, nevertheless, yield strengths sufficient for many applications. Bulk carbon nanotubes have already been used as composite fibers in polymers to improve the mechanical, thermal and electrical properties of the bulk product.
- Easton-Bell Sports, Inc. have been in partnership with Zyvex Performance Materials, using CNT technology in a number of their bicycle components—including flat and riser handlebars, cranks, forks, seatposts, stems and aero bars.
- Zyvex Technologies has also built a 54' maritime vessel, the Piranha Unmanned Surface Vessel, as a technology demonstrator for what is possible using CNT technology. CNTs help improve the structural performance of the vessel, resulting in a lightweight 8,000 lb boat that can carry a payload of 15,000 lb over a range of 2,500 miles.[138]
- Amroy Europe Oy manufactures Hybtonite carbon nanoepoxy resins where carbon nanotubes have been chemically activated to bond to epoxy, resulting in a composite material that is 20% to 30% stronger than other composite materials. It has been used for wind turbines, marine paints and variety of sports gear such as skis, ice hockey sticks, baseball bats, hunting arrows, and surfboards.[139]
Other current applications include:
- tips for atomic force microscope probes[140]
- in tissue engineering, carbon nanotubes can act as scaffolding for bone growth[141]
There is also ongoing research in using carbon nanotubes as a scaffold for diverse microfabrication techniques.[142]
Potential applications
The strength and flexibility of carbon nanotubes makes them of potential use in controlling other nanoscale structures, which suggests they will have an important role in nanotechnology engineering. The highest tensile strength of an individual multi-walled carbon nanotube has been tested to be 63 GPa.[41] Carbon nanotubes were found in Damascus steel from the 17th century, possibly helping to account for the legendary strength of the swords made of it.[143][144] Recently, several studies have highlighted the prospect of using carbon nanotubes as building blocks to fabricate three-dimensional macroscopic (>1mm in all three dimensions) all-carbon devices. Lalwani et al. have reported a novel radical initiated thermal crosslinking method to fabricated macroscopic, free-standing, porous, all-carbon scaffolds using single- and multi-walled carbon nanotubes as building blocks.[13] These scaffolds possess macro-, micro-, and nano- structured pores and the porosity can be tailored for specific applications. These 3D all-carbon scaffolds/architectures maybe used for the fabrication of the next generation of energy storage, supercapacitors, field emission transistors, high-performance catalysis, photovoltaics, and biomedical devices and implants.
Biomedical
Researchers from Rice University and State University of New York - Stony Brook have shown that the addition of low weight % of carbon nanotubes can lead to significant improvements in the mechanical properties of biodegradable polymeric nanocomposites for applications in bone tissue engineering.[145][146] Dispersion of low weight % of graphene (~0.02 wt.%) results in significant increases in compressive and flexural mechanical properties of polymeric nanocomposites. Researchers at Rice University, Radboud University Nijmegen Medical Centre and University of California, Riverside have shown that carbon nanotubes and their polymer nanocomposites are suitable scaffold materials for bone cell proliferation[141][147] and bone formation.[148][149]
In November 2012 researchers at the American National Institute of Standards and Technology (NIST) proved that single-wall carbon nanotubes may help protect DNA molecules from damage by oxidation.[150]
A highly effective method of delivering carbon nanotubes into cells is Cell squeezing, a high-throughput vector-free microfluidic platform for intracellular delivery developed at the Massachusetts Institute of Technology in the labs of Robert S. Langer.[151]
Carbon nanotubes have furthermore been grown inside microfluidic channels for chemical analysis, based on electrochromatography. Here, the high surface-area-to-volume ratio and high hydrophobicity of CNTs are used in order to greatly decrease the analysis time of small neutral molecules that typically require large bulky equipment for analysis.[152][153]
Structural
Because of the carbon nanotube's superior mechanical properties, many structures have been proposed ranging from everyday items like clothes and sports gear to combat jackets and space elevators.[154] However, the space elevator will require further efforts in refining carbon nanotube technology, as the practical tensile strength of carbon nanotubes must be greatly improved.[43]
For perspective, outstanding breakthroughs have already been made. Pioneering work led by Ray H. Baughman at the NanoTech Institute has shown that single and multi-walled nanotubes can produce materials with toughness unmatched in the man-made and natural worlds.[155][156]

Carbon nanotubes are also a promising material as building blocks in bio-mimetic hierarchical composite materials given their exceptional mechanical properties (~1 TPa in modulus, and ~100 GPa in strength). Initial attempts to incorporate CNTs into hierarchical structures led to mechanical properties that were significantly lower than these achievable limits. Windle et al. have used an in situ chemical vapor deposition (CVD) spinning method to produce continuous CNT yarns from CVD-grown CNT aerogels.[157][158] With this technology, they fabricated CNT yarns with strengths as high as ~9 GPa at small gage lengths of ~1 mm, however, defects resulted in a reduction of specific strength to ~1 GPa at 20 mm gage length.[159][160] Espinosa et al. developed high performance DWNT-polymer composite yarns by twisting and stretching ribbons of randomly oriented bundles of DWNTs thinly coated with polymeric organic compounds. These DWNT-polymer yarns exhibited an unusually high energy to failure of ~100 J·g−1 (comparable to one of the toughest natural materials – spider silk[161]), and strength as high as ~1.4 GPa.[162] Effort is ongoing to produce CNT composites that incorporate tougher matrix materials, such as Kevlar, to further improve on the mechanical properties toward those of individual CNTs.
Because of the high mechanical strength of carbon nanotubes, research is being made into weaving them into clothes to create stab-proof and bulletproof clothing. The nanotubes would effectively stop the bullet from penetrating the body, although the bullet's kinetic energy would likely cause broken bones and internal bleeding.[163]
Electrical circuits
Nanotube-based transistors, also known as carbon nanotube field-effect transistors (CNTFETs), have been made that operate at room temperature and that are capable of digital switching using a single electron.[164] However, one major obstacle to realization of nanotubes has been the lack of technology for mass production. In 2001 IBM researchers demonstrated how metallic nanotubes can be destroyed, leaving semiconducting ones behind for use as transistors. Their process is called "constructive destruction," which includes the automatic destruction of defective nanotubes on the wafer.[165] This process, however, only gives control over the electrical properties on a statistical scale.
The potential of carbon nanotubes was demonstrated in 2003 when room-temperature ballistic transistors with ohmic metal contacts and high-k gate dielectric were reported, showing 20–30x higher ON current than state-of-the-art Si MOSFETs. This presented an important advance in the field as CNT was shown to potentially outperform Si. At the time, a major challenge was ohmic metal contact formation. In this regard, palladium, which is a high-work function metal was shown to exhibit Schottky barrier-free contacts to semiconducting nanotubes with diameters >1.7 nm.[166][167]
The first nanotube integrated memory circuit was made in 2004. One of the main challenges has been regulating the conductivity of nanotubes. Depending on subtle surface features a nanotube may act as a plain conductor or as a semiconductor. A fully automated method has however been developed to remove non-semiconductor tubes.[168]
Another way to make carbon nanotube transistors has been to use random networks of them.[169] By doing so one averages all of their electrical differences and one can produce devices in large scale at the wafer level.[170] This approach was first patented by Nanomix Inc.[171] (date of original application June 2002[172]). It was first published in the academic literature by the United States Naval Research Laboratory in 2003 through independent research work. This approach also enabled Nanomix to make the first transistor on a flexible and transparent substrate.[173][174]
Large structures of carbon nanotubes can be used for thermal management of electronic circuits. An approximately 1 mm–thick carbon nanotube layer was used as a special material to fabricate coolers, this material has very low density, ~20 times lower weight than a similar copper structure, while the cooling properties are similar for the two materials.[175]
In 2013, researchers demonstrated a Turing-complete prototype micrometer-scale computer.[176][177][178] Carbon nanotube transistors as logic-gate circuits with densities comparable to modern CMOS technology has not yet been demonstrated.
Electrical cables and wires
Wires for carrying electrical current may be fabricated from pure nanotubes and nanotube-polymer composites. It has already been demonstrated that carbon nanotube wires can successfully be used for power or data transmission.[179] Recently small wires have been fabricated with specific conductivity exceeding copper and aluminum;[180][181] these cables are the highest conductivity carbon nanotube and also highest conductivity non-metal cables. Recently, composite of carbon nanotube and copper have been shown to exhibit nearly one hundred times higher current-carrying-capacity than pure copper or gold.[182] Significantly, the electrical conductivity of such a composite is similar to pure Cu. Thus, this Carbon nanotube-copper (CNT-Cu) composite possesses the highest observed current-carrying capacity among electrical conductors. Thus for a given cross-section of electrical conductor, the CNT-Cu composite can withstand and transport one hundred times higher current compared to metals such as copper and gold.
Actuators
The exceptional electrical and mechanical properties of carbon nanotubes have made them alternatives to the traditional electrical actuators for both microscopic and macroscopic applications. Carbon nanotubes are very good conductors of both electricity and heat, and they are also very strong and elastic molecules in certain directions.
Paper batteries
A paper battery is a battery engineered to use a paper-thin sheet of cellulose (which is the major constituent of regular paper, among other things) infused with aligned carbon nanotubes.[183] The nanotubes act as electrodes; allowing the storage devices to conduct electricity. The battery, which functions as both a lithium-ion battery and a supercapacitor, can provide a long, steady power output comparable to a conventional battery, as well as a supercapacitor’s quick burst of high power—and while a conventional battery contains a number of separate components, the paper battery integrates all of the battery components in a single structure, making it more energy efficient.
Solar cells
One of the promising applications of single-walled carbon nanotubes (SWNTs) is their use in solar panels, due to their strong UV/Vis-NIR absorption characteristics. Research has shown that they can provide a sizable increase in efficiency, even at their current unoptimized state. Solar cells developed at the New Jersey Institute of Technology use a carbon nanotube complex, formed by a mixture of carbon nanotubes and carbon buckyballs (known as fullerenes) to form snake-like structures. Buckyballs trap electrons, but they can't make electrons flow. Add sunlight to excite the polymers, and the buckyballs will grab the electrons. Nanotubes, behaving like copper wires, will then be able to make the electrons or current flow.[184]
Additional research has been conducted on creating SWNT hybrid solar panels to increase the efficiency further. These hybrids are created by combining SWNT's with photo-excitable electron donors to increase the number of electrons generated. It has been found that the interaction between the photo-excited porphyrin and SWNT generates electro-hole pairs at the SWNT surfaces. This phenomenon has been observed experimentally, and contributes practically to an increase in efficiency up to 8.5%.[185]
Hydrogen storage
In addition to being able to store electrical energy, there has been some research in using carbon nanotubes to store hydrogen to be used as a fuel source. By taking advantage of the capillary effects of the small carbon nanotubes, it is possible to condense gases in high density inside single-walled nanotubes. This allows for gases, most notably hydrogen (H2), to be stored at high densities without being condensed into a liquid. Potentially, this storage method could be used on vehicles in place of gas fuel tanks for a hydrogen-powered car. A current issue regarding hydrogen-powered vehicles is the on-board storage of the fuel. Current storage methods involve cooling and condensing the H2 gas to a liquid state for storage which causes a loss of potential energy (25–45%) when compared to the energy associated with the gaseous state. Storage using SWNTs would allow one to keep the H2 in its gaseous state, thereby increasing the storage efficiency. This method allows for a volume to energy ratio slightly smaller to that of current gas powered vehicles, allowing for a slightly lower but comparable range.[186]
An area of controversy and frequent experimentation regarding the storage of hydrogen by adsorption in carbon nanotubes is the efficiency by which this process occurs. The effectiveness of hydrogen storage is integral to its use as a primary fuel source since hydrogen only contains about one fourth the energy per unit volume as gasoline. Studies however show that what is the most important is the surface area of the materials used. Hence activated carbon with surface area of 2600 m2/g can store up to 5,8% w/w. In all these carbonaceous materials, hydrogen is stored by physisorption at 70-90K.[187]
Experimental capacity
One experiment[188] sought to determine the amount of hydrogen stored in CNTs by utilizing elastic recoil detection analysis (ERDA). CNTs (primarily SWNTs) were synthesized via chemical vapor disposition (CVD) and subjected to a two-stage purification process including air oxidation and acid treatment, then formed into flat, uniform discs and exposed to pure, pressurized hydrogen at various temperatures. When the data was analyzed, it was found that the ability of CNTs to store hydrogen decreased as temperature increased. Moreover, the highest hydrogen concentration measured was ~0.18%; significantly lower than commercially viable hydrogen storage needs to be. A separate experimental work performed by using a gravimetric method also revealed the maximum hydrogen uptake capacity of CNTs to be as low as 0.2%.[189]
In another experiment,[190] CNTs were synthesized via CVD and their structure was characterized using Raman spectroscopy. Utilizing microwave digestion, the samples were exposed to different acid concentrations and different temperatures for various amounts of time in an attempt to find the optimum purification method for SWNTs of the diameter determined earlier. The purified samples were then exposed to hydrogen gas at various high pressures, and their adsorption by weight percent was plotted. The data showed that hydrogen adsorption levels of up to 3.7% are possible with a very pure sample and under the proper conditions. It is thought that microwave digestion helps improve the hydrogen adsorption capacity of the CNTs by opening up the ends, allowing access to the inner cavities of the nanotubes.
Limitations on efficient hydrogen adsorption
The biggest obstacle to efficient hydrogen storage using CNTs is the purity of the nanotubes. To achieve maximum hydrogen adsorption, there must be minimum graphene, amorphous carbon, and metallic deposits in the nanotube sample. Current methods of CNT synthesis require a purification step. However, even with pure nanotubes, the absorption capacity is only maximized under high pressures, which are undesirable in commercial fuel tanks.
Supercapacitor
MIT Research Laboratory of Electronics uses nanotubes to improve supercapacitors. The activated charcoal used in conventional ultracapacitors has many small hollow spaces of various size, which create together a large surface to store electric charge. But as charge is quantized into elementary charges, i.e. electrons, and each such elementary charge needs a minimum space, a significant fraction of the electrode surface is not available for storage because the hollow spaces are not compatible with the charge's requirements. With a nanotube electrode the spaces may be tailored to size—few too large or too small—and consequently the capacity should be increased considerably.[191]
Radar absorption
Radars work in the microwave frequency range, which can be absorbed by MWNTs. Applying the MWNTs to the aircraft would cause the radar to be absorbed and therefore seem to have a smaller radar cross-section. One such application could be to paint the nanotubes onto the plane. Recently there has been some work done at the University of Michigan regarding carbon nanotubes usefulness as stealth technology on aircraft. It has been found that in addition to the radar absorbing properties, the nanotubes neither reflect nor scatter visible light, making it essentially invisible at night, much like painting current stealth aircraft black except much more effective. Current limitations in manufacturing, however, mean that current production of nanotube-coated aircraft is not possible. One theory to overcome these current limitations is to cover small particles with the nanotubes and suspend the nanotube-covered particles in a medium such as paint, which can then be applied to a surface, like a stealth aircraft.[192]
Textile
The previous studies on the use of CNTs for textile functionalization were focused on fiber spinning for improving physical and mechanical properties.[193][194][195] Recently a great deal of attention has been focused on coating CNTs on textile fabrics. Various methods have been employed for modifying fabrics using CNTs. Shim et al. produced intelligent e-textiles for Human Biomonitoring using a polyelectrolyte-based coating with CNTs.[196] Additionally, Panhuis et al. dyed textile material by immersion in either a poly (2-methoxy aniline-5-sulfonic acid) PMAS polymer solution or PMAS-SWNT dispersion with enhanced conductivity and capacitance with a durable behavior.[197] In another study, Hu and coworkers coated single-walled carbon nanotubes with a simple “dipping and drying” process for wearable electronics and energy storage applications.[198] In the recent study, Li and coworkers using elastomeric separator and almost achieved a fully stretchable supercapacitor based on buckled single-walled carbon nanotube macrofilms. The electrospun polyurethane was used and provided sound mechanical stretchability and the whole cell achieve excellent charge-discharge cycling stability.[199] CNTs have an aligned nanotube structure and a negative surface charge. Therefore, they have similar structures to direct dyes, so the exhaustion method is applied for coating and absorbing CNTs on the fiber surface for preparing multifunctional fabric including antibacterial, electric conductive, flame retardant and electromagnetic absorbance properties.[200][201][202]
Optical power detectors
A spray-on mixture of carbon nanotubes and ceramic demonstrates unprecedented ability to resist damage while absorbing laser light. Such coatings that absorb as the energy of high-powered lasers without breaking down are essential for optical power detectors that measure the output of such lasers. These are used, for example, in military equipment for defusing unexploded mines. The composite consists of multiwall carbon nanotubes and a ceramic made of silicon, carbon and nitrogen. Including boron boosts the breakdown temperature. The nanotubes and graphene-like carbon transmit heat well, while the oxidation-resistant ceramic boosts damage resistance. Creating the coating involves dispersing the nanotubes in toluene, to which a clear liquid polymer containing boron was added. The mixture was heated to 1,100 °C (2,010 °F). The result is crushed into a fine powder, dispersed again in toluene and sprayed in a thin coat on a copper surface. The coating absorbed 97.5 percent of the light from a far-infrared laser and tolerated 15 kilowatts per square centimeter for 10 seconds. Damage tolerance is about 50 percent higher than for similar coatings, e.g., nanotubes alone and carbon paint.[203][204]
Acoustics
Carbon nanotubes have also been applied in the acoustics(such as loudspeaker and earphone). In 2008 it was shown that a sheet of nanotubes can operate as a loudspeaker if an alternating current is applied. The sound is not produced through vibration but thermoacoustically.[205][206] In 2013, a carbon nanotube (CNT) thin yarn thermoacoustic earphone together with CNT thin yarn thermoacoustic chip was demonstrated by a research group of Tsinghua-Foxconn Nanotechnology Research Center in Tsinghua University,[207] using a Si-based semi-conducting technology compatible fabrication process.
Environmental remediation
A CNT nano-structured sponge (nanosponge) containing sulfur and iron is more effective at soaking up water contaminants such as oil, fertilizers, pesticides and pharmaceuticals. Their magnetic properties make them easier to retrieve once the clean-up job is done. The sulfur and iron increases sponge size to around 2 centimetres (0.79 in). It also increases porosity due to beneficial defects, creating buoyancy and reusability. Iron, in the form of ferrocene makes the structure easier to control and enables recovery using magnets. Such nanosponges increase the absorption of the toxic organic solvent dichlorobenzene from water by 3.5 times. The sponges can absorb vegetable oil up to 150 times their initial weight and can absorb engine oil as well.[208][209]
Earlier, a magnetic boron-doped MWNT nanosponge that could absorb oil from water. The sponge was grown as a forest on a substrate via chemical vapor disposition. Boron puts kinks and elbows into the tubes as they grow and promotes the formation of covalent bonds. The nanosponges retain their elastic property after 10,000 compressions in the lab. The sponges are both superhydrophobic, forcing them to remain at the water's surface and oleophilic, drawing oil to them.[210][211]
Water Treatment
It has been shown that carbon nanotubes exhibit strong adsorption affinities to a wide range of aromatic and aliphatic contaminants in water,[212][213][214] due to their large and hydrophobic surface areas. They also showed similar adsorption capacities as activated carbons in the presence of natural organic matter.[215] As a result, they have been suggested as promising adsorbents for removal of contaminant in water and wastewater treatment systems.
Moreover, membranes made out of carbon nanotube arrays have been suggested as switchable molecular sieves, with sieving and permeation features that can be dynamically activated/deactivated by either pore size distribution (passive control) or external electrostatic fields (active control).[216]
Other applications
Carbon nanotubes have been implemented in nanoelectromechanical systems, including mechanical memory elements (NRAM being developed by Nantero Inc.) and nanoscale electric motors (see Nanomotor or Nanotube nanomotor).
Carboxyl-modified single-walled carbon nanotubes (so called zig-zag, armchair type) can act as sensors of atoms and ions of alkali metals Na, Li, K.[217] In May 2005, Nanomix Inc. placed on the market a hydrogen sensor that integrated carbon nanotubes on a silicon platform. Since then, Nanomix has been patenting many such sensor applications, such as in the field of carbon dioxide, nitrous oxide, glucose, DNA detection, etc. End of 2014, Tulane University researchers have tested Nanomix's fast and fully automated point of care diagnostic system in Sierra Leone to help for rapid testing for Ebola. Nanomix announced that a product could be launched within three to six months.
Eikos Inc of Franklin, Massachusetts and Unidym Inc. of Silicon Valley, California are developing transparent, electrically conductive films of carbon nanotubes to replace indium tin oxide (ITO). Carbon nanotube films are substantially more mechanically robust than ITO films, making them ideal for high-reliability touchscreens and flexible displays. Printable water-based inks of carbon nanotubes are desired to enable the production of these films to replace ITO.[218] Nanotube films show promise for use in displays for computers, cell phones, PDAs, and ATMs.
A nanoradio, a radio receiver consisting of a single nanotube, was demonstrated in 2007.
A flywheel made of carbon nanotubes could be spun at extremely high velocity on a floating magnetic axis in a vacuum, and potentially store energy at a density approaching that of conventional fossil fuels. Since energy can be added to and removed from flywheels very efficiently in the form of electricity, this might offer a way of storing electricity, making the electrical grid more efficient and variable power suppliers (like wind turbines) more useful in meeting energy needs. The practicality of this depends heavily upon the cost of making massive, unbroken nanotube structures, and their failure rate under stress.
Carbon nanotube springs have the potential to indefinitely store elastic potential energy at ten times the density of lithium-ion batteries with flexible charge and discharge rates and extremely high cycling durability.
Ultra-short SWNTs (US-tubes) have been used as nanoscaled capsules for delivering MRI contrast agents in vivo.[219]
Carbon nanotubes provide a certain potential for metal-free catalysis of inorganic and organic reactions. For instance, oxygen groups attached to the surface of carbon nanotubes have the potential to catalyze oxidative dehydrogenations[220] or selective oxidations.[221] Nitrogen-doped carbon nanotubes may replace platinum catalysts used to reduce oxygen in fuel cells. A forest of vertically aligned nanotubes can reduce oxygen in alkaline solution more effectively than platinum, which has been used in such applications since the 1960s. Here, the nanotubes have the added benefit of not being subject to carbon monoxide poisoning.[222]
Wake Forest University engineers are using multiwalled carbon nanotubes to enhance the brightness of field-induced polymer electroluminescent technology, potentially offering a step forward in the search for safe, pleasing, high-efficiency lighting. In this technology, moldable polymer matrix emits light when exposed to an electrical current. It could eventually yield high-efficiency lights without the mercury vapor of compact fluorescent lamps or the bluish tint of some fluorescents and LEDs, which has been linked with circadian rhythm disruption.[223]
Candida albicans has been used in combination with carbon nanotubes (CNT) to produce stable electrically conductive bio-nano-composite tissue materials that have been used as temperature sensing elements.[224]
The SWNT production company OCSiAl developed a series of masterbatches for industrial use of single-wall CNTs in multiple types of rubber blends and tires, with initial trials showing increases in hardness, viscosity, tensile strain resistance and resistance to abrasion while reducing elongation and compression[225] In tires the three primary characteristics of durability, fuel efficiency and traction were improved using SWNTs. The development of rubber masterbatches built on earlier work by the Japanese National Institute of Advanced Industrial Science & Technology showing rubber to be a viable candidate for improvement with SWNTs.[226]
Popular Culture
The Tom Sloan cartoon "Carbon Nanotubes", features the attempted use of such a strong material from anewdomain.net.
Discovery
A 2006 editorial written by Marc Monthioux and Vladimir Kuznetsov in the journal Carbon described the interesting and often-misstated origin of the carbon nanotube. A large percentage of academic and popular literature attributes the discovery of hollow, nanometer-size tubes composed of graphitic carbon to Sumio Iijima of NEC in 1991.[227]
In 1952 L. V. Radushkevich and V. M. Lukyanovich published clear images of 50 nanometer diameter tubes made of carbon in the Soviet Journal of Physical Chemistry.[84] This discovery was largely unnoticed, as the article was published in Russian, and Western scientists' access to Soviet press was limited during the Cold War. It is likely that carbon nanotubes were produced before this date, but it was almost impossible to see them, as the transmission electron microscope (TEM) was not invented. When it was invented, during that time, it allowed direct visualization of these structures.
Carbon nanotubes have been produced and observed under a variety of conditions prior to 1991. A paper by Oberlin, Endo, and Koyama published in 1976 clearly showed hollow carbon fibers with nanometer-scale diameters using a vapor-growth technique.[228] Additionally, the authors show a TEM image of a nanotube consisting of a single wall of graphene. Later, Endo has referred to this image as a single-walled nanotube.[229]
In 1979, John Abrahamson presented evidence of carbon nanotubes at the 14th Biennial Conference of Carbon at Pennsylvania State University. The conference paper described carbon nanotubes as carbon fibers that were produced on carbon anodes during arc discharge. A characterization of these fibers was given as well as hypotheses for their growth in a nitrogen atmosphere at low pressures.[230]
In 1981, a group of Soviet scientists published the results of chemical and structural characterization of carbon nanoparticles produced by a thermocatalytical disproportionation of carbon monoxide. Using TEM images and XRD patterns, the authors suggested that their “carbon multi-layer tubular crystals” were formed by rolling graphene layers into cylinders. They speculated that by rolling graphene layers into a cylinder, many different arrangements of graphene hexagonal nets are possible. They suggested two possibilities of such arrangements: circular arrangement (armchair nanotube) and a spiral, helical arrangement (chiral tube).[231]
In 1987, Howard G. Tennett of Hyperion Catalysis was issued a U.S. patent for the production of "cylindrical discrete carbon fibrils" with a "constant diameter between about 3.5 and about 70 nanometers..., length 102 times the diameter, and an outer region of multiple essentially continuous layers of ordered carbon atoms and a distinct inner core...."[232]
Iijima's discovery of multi-walled carbon nanotubes in the insoluble material of arc-burned graphite rods in 1991[233] and Mintmire, Dunlap, and White's independent prediction that if single-walled carbon nanotubes could be made, then they would exhibit remarkable conducting properties[234] helped create the initial buzz that is now associated with carbon nanotubes. Nanotube research accelerated greatly following the independent discoveries[235][236] by Bethune at IBM[237] and Iijima at NEC of single-walled carbon nanotubes and methods to specifically produce them by adding transition-metal catalysts to the carbon in an arc discharge. The arc discharge technique was well-known to produce the famed Buckminster fullerene on a preparative scale,[238] and these results appeared to extend the run of accidental discoveries relating to fullerenes. The original observation of fullerenes in mass spectrometry was not anticipated,[239] and the first mass-production technique by Krätschmer and Huffman was used for several years before realizing that it produced fullerenes.[238]
The discovery of nanotubes remains a contentious issue. Many believe that Iijima's report in 1991 is of particular importance because it brought carbon nanotubes into the awareness of the scientific community as a whole.[227]
See also
- Boron nitride nanotube
- Buckypaper
- Carbide-derived carbon
- Carbon nanocone
- Carbon nanofibers
- Carbon nanoparticles
- Carbon nanoscrolls
- Carbon nanotube chemistry
- Colossal carbon tube
- Diamond nanothread
- Filamentous carbon
- Graphene oxide paper
- List of software for nanostructures modeling
- Molecular modelling
- Nanoflower
- Ninithi (nanotube modelling software)
- Organic semiconductor
- Selective chemistry of single-walled nanotubes
- Silicon nanotubes
- Timeline of carbon nanotubes
- Vantablack, a substance produced in 2014; the blackest substance known
References
This article incorporates public domain text from National Institute of Environmental Health Sciences (NIEHS) as quoted.
- ↑ 1.0 1.1 Wang, X.; Li, Qunqing; Xie, Jing; Jin, Zhong; Wang, Jinyong; Li, Yan; Jiang, Kaili; Fan, Shoushan (2009). "Fabrication of Ultralong and Electrically Uniform Single-Walled Carbon Nanotubes on Clean Substrates". Nano Letters 9 (9): 3137–3141. Bibcode:2009NanoL...9.3137W. doi:10.1021/nl901260b. PMID 19650638.
- ↑ http://news.nationalgeographic.com/news/2006/11/061116-nanotech-swords.html
- ↑ Gullapalli, S.; Wong, M.S. (2011). "Nanotechnology: A Guide to Nano-Objects" (PDF). Chemical Engineering Progress 107 (5): 28–32.
- ↑ Mintmire, J.W.; Dunlap, B.I.; White, C.T. (1992). "Are Fullerene Tubules Metallic?". Phys. Rev. Lett. 68 (5): 631–634. Bibcode:1992PhRvL..68..631M. doi:10.1103/PhysRevLett.68.631. PMID 10045950.
- ↑ Dekker, C. (1999). "Carbon nanotubes as molecular quantum wires". Physics Today 52 (5): 22–28. Bibcode:1999PhT....52e..22D. doi:10.1063/1.882658.
- ↑ Martel, R.; Derycke, V.; Lavoie, C.; Appenzeller, J.; Chan, K.; Tersoff, J.; Avouris, Ph. (2001). "Ambipolar Electrical Transport in Semiconducting Single-Wall Carbon Nanotubes". Phys. Rev. Lett. 87 (25): 256805. Bibcode:2001PhRvL..87y6805M. doi:10.1103/PhysRevLett.87.256805. PMID 11736597.
- ↑ Flahaut, E.; Bacsa, Revathi; Peigney, Alain; Laurent, Christophe (2003). "Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes". Chemical Communications 12 (12): 1442–1443. doi:10.1039/b301514a. PMID 12841282.
- ↑ Cumings, J.; Zettl, A. (2000). "Low-Friction Nanoscale Linear Bearing Realized from Multiwall Carbon Nanotubes". Science 289 (5479): 602–604. Bibcode:2000Sci...289..602C. doi:10.1126/science.289.5479.602. PMID 10915618.
- ↑ Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. (1996). "Exceptionally high Young's modulus observed for individual carbon nanotubes". Nature 381 (6584): 678–680. Bibcode:1996Natur.381..678T. doi:10.1038/381678a0.
- ↑ Zavalniuk, V.; Marchenko, S. (2011). "Theoretical analysis of telescopic oscillations in multi-walled carbon nanotubes". Low Temperature Physics 37 (4): 337. arXiv:0903.2461. Bibcode:2011LTP....37..337Z. doi:10.1063/1.3592692.
- ↑ 11.0 11.1 Liu, L.; Guo, G.; Jayanthi, C.; Wu, S. (2002). "Colossal Paramagnetic Moments in Metallic Carbon Nanotori". Phys. Rev. Lett. 88 (21): 217206. Bibcode:2002PhRvL..88u7206L. doi:10.1103/PhysRevLett.88.217206. PMID 12059501.
- ↑ Huhtala, M.; Kuronen, A.; Kaski, K. (2002). "Carbon nanotube structures: Molecular dynamics simulation at realistic limit" (PDF). Computer Physics Communications 146 (1): 30–37. Bibcode:2002CoPhC.146...30H. doi:10.1016/S0010-4655(02)00432-0.
- ↑ 13.0 13.1 Balaji Sitharaman., Lalwani, Gaurav, Andrea Trinward Kwaczala, Shruti Kanakia, Sunny C. Patel, Stefan Judex (2013). "Fabrication and characterization of three-dimensional macroscopic all-carbon scaffolds.". Carbon 53: 90–100. doi:10.1016/j.carbon.2012.10.035. PMC 3578711. PMID 23436939.
- ↑ Poelma, R.H. (17 July 2014). "Tailoring the Mechanical Properties of High-Aspect-Ratio Carbon Nanotube Arrays using Amorphous Silicon Carbide Coatings". Advanced Functional Materials 24 (36): 5737–5744. doi:10.1002/adfm.201400693.
- ↑ Yu, Kehan; Ganhua Lu; Zheng Bo; Shun Mao; Junhong Chen (2011). "Carbon Nanotube with Chemically Bonded Graphene Leaves for Electronic and Optoelectronic Applications". J. Phys. Chem. Lett. 13 2 (13): 1556–1562. doi:10.1021/jz200641c.
- ↑ Stoner, Brian R.; Akshay S. Raut; Billyde Brown; Charles B. Parker; Jeffrey T. Glass (2011). "Graphenated carbon nanotubes for enhanced electrochemical double layer capacitor performance". Appl. Phys. Lett. 18 99 (18): 183104. Bibcode:2011ApPhL..99r3104S. doi:10.1063/1.3657514.
- ↑ Hsu, Hsin-Cheng; Wang, Chen-Hao; Nataraj, S.K.; Huang, Hsin-Chih; Du, He-Yun; Chang, Sun-Tang; Chen, Li-Chyong; Chen, Kuei-Hsien (2012). "Stand-up structure of graphene-like carbon nanowalls on CNT directly grown on polyacrylonitrile-based carbon fiber paper as supercapacitor". Diamond and Related Materials 25: 176–9. doi:10.1016/j.diamond.2012.02.020.
- ↑ Parker, Charles B.; Akshay S. Raut; Billyde Brown; Brian R. Stoner; Jeffrey T. Glass (2012). "Three-dimensional arrays of graphenated carbon nanotubes". J. Mater. Res. 7 27 (7): 1046–53. Bibcode:2012JMatR..27.1046P. doi:10.1557/jmr.2012.43.
- ↑ Cui, Hong-tao; O. Zhou; B. R. Stoner (2000). "Deposition of aligned bamboo-like carbon nanotubes via microwave plasma enhanced chemical vapor deposition". J. Appl. Phys. 88 (10): 6072–4. Bibcode:2000JAP....88.6072C. doi:10.1063/1.1320024.
- ↑ Stoner, Brian R.; Jeffrey T. Glass (2012). "Carbon nanostructures: a morphological classification for charge density optimization". Diamond and Related Materials 23: 130–4. Bibcode:2012DRM....23..130S. doi:10.1016/j.diamond.2012.01.034.
- ↑ Kouvetakis, J.; Todd, M.; Wilkens, B.; Bandari, A.; Cave, N. (1994). "Novel Synthetic Routes to Carbon-Nitrogen Thin Films". Chemistry of Materials 6 (6): 811. doi:10.1021/cm00042a018.
- ↑ 22.0 22.1 Zhong, Y.; Jaidann, M.; Zhang, Y.; Zhang, G.; Liu, H.; Ioan Ionescu, M.; Li, R.; Sun, X.; Abou-Rachid, H.; Lussier, L. S. (2010). "Synthesis of high nitrogen doping of carbon nanotubes and modeling the stabilization of filled DAATO@CNTs (10,10) for nanoenergetic materials". Journal of Physics and Chemistry of Solids 71 (2): 134. doi:10.1016/j.jpcs.2009.07.030.
- ↑ Yin, L. -W.; Bando, Y.; Li, M. -S.; Liu, Y. -X.; Qi, Y. -X. (2003). "Unique Single-Crystalline Beta Carbon Nitride Nanorods". Advanced Materials 15 (21): 1840. doi:10.1002/adma.200305307.
- ↑ Oku, T.; Kawaguchi, M. (2000). "Microstructure analysis of CN-based nanocage materials by high-resolution electron microscopy". Diamond and Related Materials 9 (3–6): 906. doi:10.1016/S0925-9635(99)00359-3.
- ↑ Guo, Q.; Xie, Y.; Wang, X.; Zhang, S.; Hou, T.; Lv, S. (2004). "Synthesis of carbon nitride nanotubes with the C3N4 stoichiometry via a benzene-thermal process at low temperatures Electronic Supplementary Information (ESI) available: XRD patterns. See http://www.rsc.org/suppdata/cc/b3/b311390f/". Chemical Communications: 26. doi:10.1039/B311390F.
- ↑ Shin, W. H.; Jeong, H. M.; Kim, B. G.; Kang, J. K.; Choi, J. W. (2012). "Nitrogen-Doped Multiwall Carbon Nanotubes for Lithium Storage with Extremely High Capacity". Nano Letters 12 (5): 2283–8. doi:10.1021/nl3000908. PMID 22452675.
- ↑ "Doped nanotubes boost lithium battery power three-fold." The Register. 14 February 2013.
- ↑ Smith, Brian W.; Monthioux, Marc; Luzzi, David E. (1998). "Encapsulated C-60 in carbon nanotubes". Nature 396 (6709): 323–324. Bibcode:1998Natur.396R.323S. doi:10.1038/24521.
- ↑ Smith, B.W.; Luzzi, D.E. (2000). "Formation mechanism of fullerene peapods and coaxial tubes: a path to large scale synthesis". Chem. Phys. Lett. 321 (1-2): 169–174. Bibcode:2000CPL...321..169S. doi:10.1016/S0009-2614(00)00307-9.
- ↑ Su, H.; Goddard, W.A.; Zhao, Y. (2006). "Dynamic friction force in a carbon peapod oscillator". Nanotechnology 17 (22): 5691–5695. arXiv:cond-mat/0611671. Bibcode:2006Nanot..17.5691S. doi:10.1088/0957-4484/17/22/026.
- ↑ Wang, M.; Li, C.M. (2010). "An oscillator in a carbon peapod controllable by an external electric field: A molecular dynamics study". Nanotechnology 21 (3): 035704. Bibcode:2010Nanot..21c5704W. doi:10.1088/0957-4484/21/3/035704.
- ↑ Liu, Q.; Ren, Wencai; Chen, Zhi-Gang; Yin, Lichang; Li, Feng; Cong, Hongtao; Cheng, Hui-Ming (2009). "Semiconducting properties of cup-stacked carbon nanotubes" (PDF). Carbon 47 (3): 731–736. doi:10.1016/j.carbon.2008.11.005.
- ↑ Zhang, R.; Zhang, Y.; Zhang, Q.; Xie, H.; Qian, W.; Wei, F. (2013). "Growth of Half-Meter Long Carbon Nanotubes Based on Schulz–Flory Distribution". ACS Nano 7 (7): 6156–61. doi:10.1021/nn401995z. PMID 23806050.
- ↑ "A Better Way to Make Nanotubes". Lawrence Berkeley National Laboratory. January 5, 2009.
- ↑ Bertozzi, C. (2009). "Carbon Nanohoops: Shortest Segment of a Carbon Nanotube Synthesized" (PDF). Lawrence Berkeley National Laboratory.
- ↑ Zhao, X.; Liu, Y.; Inoue, S.; Suzuki, T.; Jones, R.; Ando, Y. (2004). "Smallest Carbon Nanotube is 3 Å in Diameter". Phys. Rev. Lett. 92 (12): 125502. Bibcode:2004PhRvL..92l5502Z. doi:10.1103/PhysRevLett.92.125502. PMID 15089683.
- ↑ Hayashi, Takuya; Kim, Yoong Ahm; Matoba, Toshiharu; Esaka, Masaya; Nishimura, Kunio; Tsukada, Takayuki; Endo, Morinobu; Dresselhaus, Mildred S. (2003). "Smallest Freestanding Single-Walled Carbon Nanotube". Nano Letters 3 (7): 887–889. Bibcode:2003NanoL...3..887H. doi:10.1021/nl034080r.
- ↑ Guan, L.; Suenaga, K.; Iijima, S. (2008). "Smallest Carbon Nanotube Assigned with Atomic Resolution Accuracy". Nano Letters 8 (2): 459–462. Bibcode:2008NanoL...8..459G. doi:10.1021/nl072396j. PMID 18186659.
- ↑ "Densest array of carbon nanotubes grown to date". KurzweilAI. 2013-09-27.
- ↑ Sugime, H.; Esconjauregui, S.; Yang, J.; d'Arsié, L.; Oliver, R. A.; Bhardwaj, S.; Cepek, C.; Robertson, J. (2013). "Low temperature growth of ultra-high mass density carbon nanotube forests on conductive supports". Applied Physics Letters 103 (7): 073116. doi:10.1063/1.4818619.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 Yu, M.-F.; Lourie, O; Dyer, MJ; Moloni, K; Kelly, TF; Ruoff, RS (2000). "Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load". Science 287 (5453): 637–640. Bibcode:2000Sci...287..637Y. doi:10.1126/science.287.5453.637. PMID 10649994.
- ↑ 42.0 42.1 Peng, B.; Locascio, Mark; Zapol, Peter; Li, Shuyou; Mielke, Steven L.; Schatz, George C.; Espinosa, Horacio D. (2008). "Measurements of near-ultimate strength for multiwalled carbon nanotubes and irradiation-induced crosslinking improvements". Nature Nanotechnology 3 (10): 626–631. doi:10.1038/nnano.2008.211. PMID 18839003.
- ↑ 43.0 43.1 43.2 43.3 Collins, P.G. (2000). "Nanotubes for Electronics". Scientific American: 67–69.
- ↑ 44.0 44.1 Filleter, T.; Bernal, R.; Li, S.; Espinosa, H.D. (2011). "Ultrahigh Strength and Stiffness in Cross-Linked Hierarchical Carbon Nanotube Bundles". Advanced Materials 23 (25): 2855–2860. doi:10.1002/adma.201100547.
- ↑ Jensen, K.; Mickelson, W.; Kis, A.; Zettl, A. (2007). "Buckling and kinking force measurements on individual multiwalled carbon nanotubes". Physical Review B 76 (19): 195436. Bibcode:2007PhRvB..76s5436J. doi:10.1103/PhysRevB.76.195436.
- ↑ Bellucci, S. (2005). "Carbon nanotubes: Physics and applications". Physica Status Solidi (c) 2 (1): 34–47. Bibcode:2005PSSCR...2...34B. doi:10.1002/pssc.200460105.
- ↑ Chae, H.G.; Kumar, S. (2006). "Rigid Rod Polymeric Fibers". Journal of Applied Polymer Science 100 (1): 791–802. doi:10.1002/app.22680.
- ↑ Meo, M.; Rossi, M. (2006). "Prediction of Young's modulus of single wall carbon nanotubes by molecular-mechanics-based finite element modelling". Composites Science and Technology 66 (11–12): 1597–1605. doi:10.1016/j.compscitech.2005.11.015.
- ↑ Sinnott, S.B.; Andrews, R. (2001). "Carbon Nanotubes: Synthesis, Properties, and Applications". Critical Reviews in Solid State and Materials Sciences 26 (3): 145–249. Bibcode:2001CRSSM..26..145S. doi:10.1080/20014091104189.
- ↑ 50.0 50.1 Demczyk, B.G.; Wang, Y.M; Cumings, J; Hetman, M; Han, W; Zettl, A; Ritchie, R.O (2002). "Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes". Materials Science and Engineering A 334 (1–2): 173–178. doi:10.1016/S0921-5093(01)01807-X.
- ↑ 51.0 51.1 "Properties of Stainless Steel". Australian Stainless Steel Development Association.
- ↑ 52.0 52.1 "Stainless Steel – 17-7PH (Fe/Cr17/Ni 7) Material Information".
- ↑ 53.0 53.1 Wagner, H.D. (2002). "Reinforcement". Encyclopedia of Polymer Science and Technology. John Wiley & Sons. doi:10.1002/0471440264.pst317.
- ↑ Ruoff, R. S.; Tersoff, J.; Lorents, D. C.; Subramoney, S.; Chan, B. (1993). "Radial deformation of carbon nanotubes by van der Waals forces". Nature 364 (6437): 514. doi:10.1038/364514a0.
- ↑ Palaci, I.; Fedrigo, S.; Brune, H.; Klinke, C.; Chen, M.; Riedo, E. (2005). "Radial Elasticity of Multiwalled Carbon Nanotubes". Physical Review Letters 94 (17). doi:10.1103/PhysRevLett.94.175502.
- ↑ Yu, M. F.; Kowalewski, T.; Ruoff, R. (2000). "Investigation of the Radial Deformability of Individual Carbon Nanotubes under Controlled Indentation Force". Physical Review Letters 85 (7): 1456–9. doi:10.1103/PhysRevLett.85.1456. PMID 10970528.
- ↑ Yang, Y. H.; Li, W. Z. (2011). "Radial elasticity of single-walled carbon nanotube measured by atomic force microscopy". Applied Physics Letters 98 (4): 041901. doi:10.1063/1.3546170.
- ↑ Popov, M.; Kyotani, M.; Nemanich, R.; Koga, Y. (2002). "Superhard phase composed of single-wall carbon nanotubes" (PDF). Phys. Rev. B 65 (3): 033408. Bibcode:2002PhRvB..65c3408P. doi:10.1103/PhysRevB.65.033408.
- ↑ Sanders, R. (March 23, 2003). "Physicists build world's smallest motor using nanotubes and etched silicon" (Press release). UC Berkeley.
- ↑ 60.0 60.1 Lu, X.; Chen, Z. (2005). "Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (C60) and Single-Walled Carbon Nanotubes". Chemical Reviews 105 (10): 3643–3696. doi:10.1021/cr030093d. PMID 16218563.
- ↑ Hong, Seunghun; Myung, S (2007). "Nanotube Electronics: A flexible approach to mobility". Nature Nanotechnology 2 (4): 207–208. Bibcode:2007NatNa...2..207H. doi:10.1038/nnano.2007.89. PMID 18654263.
- ↑ Charlier, J. C.; Roche, S. (2007). "Electronic and transport properties of nanotubes". Reviews of Modern Physics 79 (2): 677. doi:10.1103/RevModPhys.79.677.
- ↑ Tang, Z. K.; Zhang, L; Wang, N; Zhang, XX; Wen, GH; Li, GD; Wang, JN; Chan, CT; Sheng, P (2001). "Superconductivity in 4 Angstrom Single-Walled Carbon Nanotubes". Science 292 (5526): 2462–5. Bibcode:2001Sci...292.2462T. doi:10.1126/science.1060470. PMID 11431560.
Takesue, I.; Haruyama, J.; Kobayashi, N.; Chiashi, S.; Maruyama, S.; Sugai, T.; Shinohara, H. (2006). "Superconductivity in Entirely End-Bonded Multiwalled Carbon Nanotubes" (PDF). Phys. Rev. Lett. 96 (5): 057001. arXiv:cond-mat/0509466. Bibcode:2006PhRvL..96e7001T. doi:10.1103/PhysRevLett.96.057001. PMID 16486971.
Lortz, R.; Zhang, Q; Shi, W; Ye, J. T.; Qiu, C. Y.; Wang, Z.; He, H. T.; Sheng, P; Qian, T. Z.; Tang, Z. K.; Wang, N.; Zhang, X. X.; Wang, J; Chan, C. T. (2009). "Superconducting characteristics of 4-A carbon nanotube–zeolite composite". Proceedings of the National Academy of Sciences 106 (18): 7299–7303. Bibcode:2009PNAS..106.7299L. doi:10.1073/pnas.0813162106. - ↑ M. Bockrath (2006). "Carbon nanotubes: The weakest link". Nature Physics 2 (3): 155–156. Bibcode:2006NatPh...2..155B. doi:10.1038/nphys252.
- ↑ Pop, Eric; Mann, David; Wang, Qian; Goodson, Kenneth; Dai, Hongjie (2005-12-22). "Thermal conductance of an individual single-wall carbon nanotube above room temperature". Nano Letters 6 (1): 96–100. arXiv:cond-mat/0512624. Bibcode:2006NanoL...6...96P. doi:10.1021/nl052145f. PMID 16402794.
- ↑ Sinha, Saion; Barjami, Saimir; Iannacchione, Germano; Schwab, Alexander; Muench, George (2005-06-05). "Off-axis thermal properties of carbon nanotube films". Journal of Nanoparticle Research 7 (6): 651–657. doi:10.1007/s11051-005-8382-9.
- ↑ Thostenson, Erik; Li, C; Chou, T (2005). "Nanocomposites in context". Composites Science and Technology 65 (3–4): 491–516. doi:10.1016/j.compscitech.2004.11.003.
- ↑ Carbon-Based Magnetism: An Overview of the Magnetism of Metal Free Carbon-based Compounds and Materials, Tatiana Makarova and Fernando Palacio (eds.), Elsevier, 2006
- ↑ Mingo, N.; Stewart, D. A.; Broido, D. A.; Srivastava, D. (2008). "Phonon transmission through defects in carbon nanotubes from first principles". Phys. Rev. B 77 (3): 033418. Bibcode:2008PhRvB..77c3418M. doi:10.1103/PhysRevB.77.033418.
- ↑ 70.0 70.1 Kolosnjaj J, Szwarc H, Moussa F (2007). "Toxicity studies of carbon nanotubes". Adv Exp Med Biol. Advances in Experimental Medicine and Biology 620: 181–204. doi:10.1007/978-0-387-76713-0_14. ISBN 978-0-387-76712-3. PMID 18217344.
- ↑ Corredor, C.; Hou, W.C.; Klein, S.A.; Moghadam, B.Y.; Goryll, M.; Doudrick, K.; Westerhoff, P.; Posner, J.D. (2013). "Disruption of model cell membranes by carbon nanotubes". Carbon 60: 67–75. doi:10.1016/j.carbon.2013.03.057.
- ↑ 72.0 72.1 Porter, Alexandra; Gass, Mhairi; Muller, Karin; Skepper, Jeremy N.; Midgley, Paul A.; Welland, Mark (2007). "Direct imaging of single-walled carbon nanotubes in cells". Nature Nanotechnology 2 (11): 713–7. Bibcode:2007NatNa...2..713P. doi:10.1038/nnano.2007.347. PMID 18654411.
- ↑ Zumwalde, Ralph and Laura Hodson (March 2009). "Approaches to Safe Nanotechnology: Managing the Health and Safety Concerns Associated with Engineered Nanomaterials". National Institute for Occupational Safety and Health. NIOSH (DHHS) Publication 2009-125.
- ↑ 74.0 74.1 Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL (2006). "A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks". Crit Rev Toxicol. 36 (3): 189–217. doi:10.1080/10408440600570233. PMID 16686422.
- ↑ James D Byrne; John A Baugh (2008). "The significance of nano particles in particle-induced pulmonary fibrosis". McGill Journal of Medicine 11 (1): 43–50. PMC 2322933. PMID 18523535.
- ↑ 76.0 76.1 76.2 Poland, CA; Duffin, Rodger; Kinloch, Ian; Maynard, Andrew; Wallace, William A. H.; Seaton, Anthony; Stone, Vicki; Brown, Simon et al. (2008). "Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study". Nature Nanotechnology 3 (7): 423–8. doi:10.1038/nnano.2008.111. PMID 18654567.
- ↑ Iijima, Sumio (1991). "Helical microtubules of graphitic carbon". Nature 354 (6348): 56–58. Bibcode:1991Natur.354...56I. doi:10.1038/354056a0.
- ↑ Ebbesen, T. W.; Ajayan, P. M. (1992). "Large-scale synthesis of carbon nanotubes". Nature 358 (6383): 220–222. Bibcode:1992Natur.358..220E. doi:10.1038/358220a0.
- ↑ Guo, Ting; Nikolaev, Pavel; Rinzler, Andrew G.; Tomanek, David; Colbert, Daniel T.; Smalley, Richard E. (1995). "Self-Assembly of Tubular Fullerenes" (PDF). J. Phys. Chem. 99 (27): 10694–10697. doi:10.1021/j100027a002.
- ↑ Guo, Ting; Nikolaev, P; Thess, A; Colbert, D; Smalley, R (1995). "Catalytic growth of single-walled nanotubes by laser vaporization" (PDF). Chem. Phys. Lett. 243 (1-2): 49–54. Bibcode:1995CPL...243...49B. doi:10.1016/0009-2614(95)00825-O.
- ↑ Smiljanic, Olivier; Stansfield, B.L.; Dodelet, J.-P.; Serventi, A.; Désilets, S. (22 April 2002). "Gas-phase synthesis of SWNT by an atmospheric pressure plasma jet". Chemical Physics Letters 356 (3–4): 189–193. Bibcode:2002CPL...356..189S. doi:10.1016/S0009-2614(02)00132-X.
- ↑ Smiljanic, Olivier. "Method and apparatus for producing single-wall carbon nanotubes". US Patent.
- ↑ Kim, K.S.; Cota-Sanchez, German; Kingston, Chris; Imris, M.; Simard, Benoît; Soucy, Gervais (2007). "Large-scale production of single-wall carbon nanotubes by induction thermal plasma". Journal of Physics D: Applied Physics 40 (8): 2375–2387. Bibcode:2007JPhD...40.2375K. doi:10.1088/0022-3727/40/8/S17.
- ↑ 84.0 84.1 Радушкевич, Л. В. (1952). О Структуре Углерода, Образующегося При Термическом Разложении Окиси Углерода На Железном Контакте (PDF). Журнал Физической Химии (in Russian) 26: 88–95.
- ↑ Walker Jr., P. L.; Rakszawski, J. F.; Imperial, G. R. (1959). "Carbon Formation from Carbon Monoxide-Hydrogen Mixtures over Iron Catalysts. I. Properties of Carbon Formed". J. Phys. Chem. 63 (2): 133–140. doi:10.1021/j150572a002.
- ↑ José-Yacamán, M.; Miki-Yoshida, M.; Rendón, L.; Santiesteban, J. G. (1993). "Catalytic growth of carbon microtubules with fullerene structure". Appl. Phys. Lett. 62 (6): 657. Bibcode:1993ApPhL..62..657J. doi:10.1063/1.108857.
- ↑ Beckman, Wendy (2007-04-27). "UC Researchers Shatter World Records with Length of Carbon Nanotube Arrays". University of Cincinnati.
- ↑ Inami, Nobuhito; Ambri Mohamed, Mohd; Shikoh, Eiji; Fujiwara, Akihiko (2007). "Synthesis-condition dependence of carbon nanotube growth by alcohol catalytic chemical vapor deposition method" (PDF). Sci. Technol. Adv. Mater. 8 (4): 292–295. Bibcode:2007STAdM...8..292I. doi:10.1016/j.stam.2007.02.009.
- ↑ N. Ishigami; Ago, H; Imamoto, K; Tsuji, M; Iakoubovskii, K; Minami, N (2008). "Crystal Plane Dependent Growth of Aligned Single-Walled Carbon Nanotubes on Sapphire". J. Am. Chem. Soc. 130 (30): 9918–9924. doi:10.1021/ja8024752. PMID 18597459.
- ↑ Naha, Sayangdev, and Ishwar K. Puri (2008). "A model for catalytic growth of carbon nanotubes". Journal of Physics D: Applied Physics 41 (6): 065304. Bibcode:2008JPhD...41f5304N. doi:10.1088/0022-3727/41/6/065304.
- ↑ Banerjee, Soumik, Naha, Sayangdev, and Ishwar K. Puri (2008). "Molecular simulation of the carbon nanotube growth mode during catalytic synthesis". Applied Physics Letters 92 (23): 233121. Bibcode:2008ApPhL..92w3121B. doi:10.1063/1.2945798.
- ↑ Pinilla, JL; Moliner, R; Suelves, I; Lazaro, M; Echegoyen, Y; Palacios, J (2007). "Production of hydrogen and carbon nanofibers by thermal decomposition of methane using metal catalysts in a fluidized bed reactor". International Journal of Hydrogen Energy 32 (18): 4821–4829. doi:10.1016/j.ijhydene.2007.08.013.
- ↑ Muradov, N (2001). "Hydrogen via methane decomposition: an application for decarbonization of fossil fuels". International Journal of Hydrogen Energy 26 (11): 1165–1175. doi:10.1016/S0360-3199(01)00073-8.
- ↑ Kumar, M. (2010). "Chemical vapor deposition of carbon nanotubes: a review on growth mechanism and mass production.". Journal of Nanoscience and Nanotechnology 10 (6): 6. doi:10.1166/jnn.2010.2939.
- ↑ Eftekhari, A.; Jafarkhani, P; Moztarzadeh, F (2006). "High-yield synthesis of carbon nanotubes using a water-soluble catalyst support in catalytic chemical vapor deposition". Carbon 44 (7): 1343–1345. doi:10.1016/j.carbon.2005.12.006.
- ↑ Ren, Z. F.; Huang, ZP; Xu, JW; Wang, JH; Bush, P; Siegal, MP; Provencio, PN (1998). "Synthesis of Large Arrays of Well-Aligned Carbon Nanotubes on Glass". Science 282 (5391): 1105–7. Bibcode:1998Sci...282.1105R. doi:10.1126/science.282.5391.1105. PMID 9804545.
- ↑ SEM images & TEM images of carbon nanotubes, aligned carbon nanotube arrays, and nanoparticles. Nano-lab.com.
- ↑ Neupane, Suman; Lastres, Mauricio; Chiarella, M; Li, W.Z.; Su, Q; Du, G.H. (2012). "Synthesis and field emission properties of vertically aligned carbon nanotube arrays on copper". Carbon 50 (7): 2641–50. doi:10.1016/j.carbon.2012.02.024.
- ↑ Kumar, Mukul; Ando, Yoshinori (2007). "Carbon Nanotubes from Camphor: An Environment-Friendly Nanotechnology" (PDF). Journal of Physics: Conference Series 61: 643–646. Bibcode:2007JPhCS..61..643K. doi:10.1088/1742-6596/61/1/129.
- ↑ Smalley, Richard E.; Li, Yubao; Moore, Valerie C.; Price, B. Katherine; Colorado, Ramon; Schmidt, Howard K.; Hauge, Robert H.; Barron, Andrew R.; Tour, James M. (2006). "Single Wall Carbon Nanotube Amplification: En Route to a Type-Specific Growth Mechanism". Journal of the American Chemical Society 128 (49): 15824–15829. doi:10.1021/ja065767r. ISSN 0002-7863. PMID 17147393.
- ↑ Hata, K.; Futaba, DN; Mizuno, K; Namai, T; Yumura, M; Iijima, S (2004). "Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes". Science 306 (5700): 1362–1365. Bibcode:2004Sci...306.1362H. doi:10.1126/science.1104962. PMID 15550668.
- ↑ Futaba, Don; Hata, Kenji; Yamada, Takeo; Mizuno, Kohei; Yumura, Motoo; Iijima, Sumio (2005). "Kinetics of Water-Assisted Single-Walled Carbon Nanotube Synthesis Revealed by a Time-Evolution Analysis". Phys. Rev. Lett. 95 (5): 056104. Bibcode:2005PhRvL..95e6104F. doi:10.1103/PhysRevLett.95.056104.
- ↑ Hiraoka, Tatsuki; Izadi-Najafabadi, Ali; Yamada, Takeo; Futaba, Don N.; Yasuda, Satoshi; Tanaike, Osamu; Hatori, Hiroaki; Yumura, Motoo et al. (2009). "Compact and light supercapacitors from a surface-only solid by opened carbon nanotubes with 2,200 m2/g". Advanced Functional Materials 20 (3): 422–428. doi:10.1002/adfm.200901927.
- ↑ "Unidym product sheet SWNT" (PDF).
- ↑ "Characteristic of Carbon nanotubes by super-growth method" (in Japanese).
- ↑ 106.0 106.1 K.Hata. "From Highly Efficient Impurity-Free CNT Synthesis to DWNT forests, CNTsolids and Super-Capacitors" (PDF).
- ↑ Yamada, Takeo; Namai, Tatsunori; Hata, Kenji; Futaba, Don N.; Mizuno, Kohei; Fan, Jing; Yudasaka, Masako; Yumura, Motoo; Iijima, Sumio (2006). "Size-selective growth of double-walled carbon nanotube forests from engineered iron catalysts". Nature Nanotechnology 1 (2): 131–136. Bibcode:2006NatNa...1..131Y. doi:10.1038/nnano.2006.95. PMID 18654165.
- ↑ Futaba, Don N.; Hata, Kenji; Yamada, Takeo; Hiraoka, Tatsuki; Hayamizu, Yuhei; Kakudate, Yozo; Tanaike, Osamu; Hatori, Hiroaki et al. (2006). "Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes". Nature Materials 5 (12): 987–994. Bibcode:2006NatMa...5..987F. doi:10.1038/nmat1782. PMID 17128258.
- ↑ Singer, J.M. (1959). "Carbon formation in very rich hydrocarbon-air flames. I. Studies of chemical content, temperature, ionization and particulate matter". Seventh Symposium (International) on Combustion.
- ↑ Yuan, Liming; Saito, Kozo; Pan, Chunxu; Williams, F.A; Gordon, A.S (2001). "Nanotubes from methane flames". Chemical physics letters 340 (3–4): 237–241. Bibcode:2001CPL...340..237Y. doi:10.1016/S0009-2614(01)00435-3.
- ↑ Yuan, Liming; Saito, Kozo; Hu, Wenchong; Chen, Zhi (2001). "Ethylene flame synthesis of well-aligned multi-walled carbon nanotubes". Chemical physics letters 346 (1-2): 23–28. Bibcode:2001CPL...346...23Y. doi:10.1016/S0009-2614(01)00959-9.
- ↑ Duan, H. M.; McKinnon, J. T. (1994). "Nanoclusters Produced in Flames". Journal of Physical Chemistry 98 (49): 12815–12818. doi:10.1021/j100100a001.
- ↑ Murr, L. E.; Bang, J.J.; Esquivel, E.V.; Guerrero, P.A.; Lopez, D.A. (2004). "Carbon nanotubes, nanocrystal forms, and complex nanoparticle aggregates in common fuel-gas combustion sources and the ambient air". Journal of Nanoparticle Research 6 (2/3): 241–251. doi:10.1023/B:NANO.0000034651.91325.40.
- ↑ Vander Wal, R.L. (2002). "Fe-catalyzed single-walled carbon nanotube synthesis within a flame environment". Combust. Flame 130 (1-2): 37–47. doi:10.1016/S0010-2180(02)00360-7.
- ↑ Saveliev, A.V.; Merchan-Merchan, Wilson; Kennedy, Lawrence A. (2003). "Metal catalyzed synthesis of carbon nanostructures in an opposed flow methane oxygen flame". Combust. Flame 135 (1-2): 27–33. doi:10.1016/S0010-2180(03)00142-1.
- ↑ Height, M.J.; Howard, Jack B.; Tester, Jefferson W.; Vander Sande, John B. (2004). "Flame synthesis of single-walled carbon nanotubes". Carbon 42 (11): 2295–2307. doi:10.1016/j.carbon.2004.05.010.
- ↑ Sen, S.; Puri, Ishwar K (2004). "Flame synthesis of carbon nanofibers and nanofibers composites containing encapsulated metal particles". Nanotechnology 15 (3): 264–268. Bibcode:2004Nanot..15..264S. doi:10.1088/0957-4484/15/3/005.
- ↑ Naha, Sayangdev, Sen, Swarnendu, De, Anindya K., and Puri, Ishwar K. (2007). "A detailed model for the Flame synthesis of carbon nanotubes and nanofibers". Proceedings of the Combustion Institute 31 (2): 1821–29. doi:10.1016/j.proci.2006.07.224.
- ↑ Yamada T, Namai T, Hata K, Futaba DN, Mizuno K, Fan J et al. (2006). "Size-selective growth of double-walled carbon nanotube forests from engineered iron catalysts". Nature Nanotechnology 1 (2): 131–136. Bibcode:2006NatNa...1..131Y. doi:10.1038/nnano.2006.95. PMID 18654165.
- ↑ MacKenzie KJ, Dunens OM, Harris AT (2010). "An updated review of synthesis parameters and growth mechanisms for carbon nanotubes in fluidized beds". Industrial & Engineering Chemical Research 49 (11): 5323–38. doi:10.1021/ie9019787.
- ↑ Jakubek LM, Marangoudakis S, Raingo J, Liu X, Lipscombe D, Hurt RH; Marangoudakis; Raingo; Liu; Lipscombe; Hurt (2009). "The inhibition of neuronal calcium ion channels by trace levels of yttrium released from carbon nanotubes". Biomaterials 30 (31): 6351–6357. doi:10.1016/j.biomaterials.2009.08.009. PMC 2753181. PMID 19698989.
- ↑ Hou P-X, Liu C, Cheng H-M (2008). "Purification of carbon nanotubes". Carbon 46 (15): 2003–2025. doi:10.1016/j.carbon.2008.09.009.
- ↑ Ebbesen TW, Ajayan PM, Hiura H, Tanigaki K; Ajayan; Hiura; Tanigaki (1994). "Purification of nanotubes". Nature 367 (6463): 519. Bibcode:1994Natur.367..519E. doi:10.1038/367519a0.
- ↑ Xu Y-Q, Peng H, Hauge RH, Smalley RE; Peng; Hauge; Smalley (2005). "Controlled multistep purification of single-walled carbon nanotubes". Nano Letters 5 (1): 163–168. Bibcode:2005NanoL...5..163X. doi:10.1021/nl048300s. PMID 15792432.
- ↑ Meyer-Plath A, Orts-Gil G, Petrov S et al. (2012). "Plasma-thermal purification and annealing of carbon nanotubes". Carbon 50 (10): 3934–3942. doi:10.1016/j.carbon.2012.04.049.
- ↑ 126.0 126.1 Arnold, Michael S.; Green, Alexander A.; Hulvat, James F.; Stupp, Samuel I.; Hersam, Mark C. (2006). "Sorting carbon nanotubes by electronic structure using density differentiation". Nature Nanotechnology 1 (1): 60–5. Bibcode:2006NatNa...1...60A. doi:10.1038/nnano.2006.52. PMID 18654143.
- ↑ Tanaka, Takeshi; Jin, Hehua; Miyata, Yasumitsu; Fujii, Shunjiro; Suga, Hiroshi; Naitoh, Yasuhisa; Minari, Takeo; Miyadera, Tetsuhiko et al. (2009). "Simple and Scalable Gel-Based Separation of Metallic and Semiconducting Carbon Nanotubes". Nano Letters 9 (4): 1497–1500. Bibcode:2009NanoL...9.1497T. doi:10.1021/nl8034866. PMID 19243112.
- ↑ T.Tanaka. "New, Simple Method for Separation of Metallic and Semiconducting Carbon Nanotubes".
- ↑ Yamada, Y.; Tanaka, T.; Machida, K.; Suematsu, S.; Tamamitsu, K.; Kataura, H.; Hatori, H. (2012). "Electrochemical behavior of metallic and semiconducting single-wall carbon nanotubes for electric double-layer capacitor". Carbon 50 (3): 1422. doi:10.1016/j.carbon.2011.09.062.
- ↑ Tanaka, Takeshi; Urabe, Yasuko; Nishide, Daisuke; Kataura, Hiromichi (2009). "Continuous Separation of Metallic and Semiconducting Carbon Nanotubes Using Agarose Gel". Applied Physics Express 2 (12): 125002. Bibcode:2009APExp...2l5002T. doi:10.1143/APEX.2.125002.
- ↑ Huang, Xueying; McLean, Robert S.; Zheng, Ming (2005). "High-Resolution Length Sorting and Purification of DNA-Wrapped Carbon Nanotubes by Size-Exclusion Chromatography". Anal. Chem. 77 (19): 6225–6228. doi:10.1021/ac0508954. PMID 16194082.
- ↑ Mark C Hersam (2008). "Progress towards monodisperse single-walled carbon nanotubes". Nature Nanotechnology 3 (7): 387–394. Bibcode:2008NatNa...3..387H. doi:10.1038/nnano.2008.135. PMID 18654561.
- ↑ Zheng, M.; Jagota, A; Strano, MS; Santos, AP; Barone, P; Chou, SG; Diner, BA; Dresselhaus, MS et al. (2003). "Structure-Based Carbon Nanotube Sorting by Sequence-Dependent DNA Assembly". Science 302 (5650): 1545–1548. Bibcode:2003Sci...302.1545Z. doi:10.1126/science.1091911. PMID 14645843.
- ↑ Tu, Xiaomin; Manohar, Suresh; Jagota, Anand; Zheng, Ming (2009). "DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes". Nature 460 (7252): 250–253. Bibcode:2009Natur.460..250T. doi:10.1038/nature08116. PMID 19587767.
- ↑ Zhang, Li; Tu, Xiaomin; Welsher, Kevin; Wang, Xinran; Zheng, Ming; Dai, Hongjie (2009). "Optical characterizations and electronic devices of nearly pure (10,5) single-walled carbon nanotubes". J Am Chem Soc 131 (7): 2454–2455. doi:10.1021/ja8096674. PMID 19193007.
- ↑ Ding, Lei; Tselev, Alexander; Wang, Jinyong; Yuan, Dongning; Chu, Haibin; McNicholas, Thomas P.; Li, Yan; Liu, Jie (2009). "Selective Growth of Well-Aligned Semiconducting Single-Walled Carbon Nanotubes". Nano Letters 9 (2): 800–5. Bibcode:2009NanoL...9..800D. doi:10.1021/nl803496s. PMID 19159186.
- ↑ Mohamed, Mohd Ambri; Inami, Nobuhito; Shikoh, Eiji; Yamamoto, Yoshiyuki; Hori, Hidenobu; Fujiwara, Akihiko (2008). "Fabrication of spintronics device by direct synthesis of single-walled carbon nanotubes from ferromagnetic electrodes" (PDF). Sci. Technol. Adv. Mater. 9 (2): 025019. doi:10.1088/1468-6996/9/2/025019.
- ↑ "Pirahna USV built using nano-enhanced carbon prepreg". ReinforcedPlastics.com. 19 February 2009.
- ↑ Pagni, John (5 March 2010). "Amroy aims to become nano-leader". European Plastics News.
- ↑ "Nanotube Tips". nanoScince instruments.
- ↑ 141.0 141.1 Haddon, Robert C.; Laura P. Zanello; Bin Zhao; Hui Hu (2006). "Bone Cell Proliferation on Carbon Nanotubes". Nano Letters 6 (3): 562–567. Bibcode:2006NanoL...6..562Z. doi:10.1021/nl051861e. PMID 16522063.
- ↑ "Publications on carbon nanotube applications including scaffold microfabrication". nano.byu.edu. 27 May 2014.
- ↑ K. Sanderson (2006). "Sharpest cut from nanotube sword". Nature News. doi:10.1038/news061113-11.
- ↑ Reibold, M.; Paufler, P; Levin, AA; Kochmann, W; Pätzke, N; Meyer, DC (November 16, 2006). "Materials: Carbon nanotubes in an ancient Damascus sabre". Nature 444 (7117): 286. Bibcode:2006Natur.444..286R. doi:10.1038/444286a. PMID 17108950.
- ↑ Balaji Sitharaman., Lalwani, Gaurav, Allan M. Henslee, Behzad Farshid, Liangjun Lin, F. Kurtis Kasper, Yi-Xian Qin, Antonios G. Mikos (2013). "Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering.". Biomacromolecules 14 (3): 900–909. doi:10.1021/bm301995s. PMC 3601907. PMID 23405887.
- ↑ Lalwani, Gaurav (September 2013). "Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering". Acta Biomaterialia 9 (9): 8365–8373. doi:10.1016/j.actbio.2013.05.018. PMC 3732565. PMID 23727293. PMID 23727293 . Full Text PDF
- ↑ Shi, Xinfeng; Sitharaman, Balaji; Pham, Quynh P.; Liang, Feng; Wu, Katherine; Edward Billups, W.; Wilson, Lon J.; Mikos, Antonios G. (2007). "Fabrication of porous ultra-short single-walled carbon nanotubenanocomposite scaffolds for bone tissue engineering". Biomaterials 28 (28): 4078–4090. doi:10.1016/j.biomaterials.2007.05.033. PMC 3163100. PMID 17576009.
- ↑ Sitharaman, Balaji; Shi, Xinfeng; Walboomers, X. Frank; Liao, Hongbing; Cuijpers, Vincent; Wilson, Lon J.; Mikos, Antonios G.; Jansen, John A. (2008). "In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering". Bone 43 (2): 362–370. doi:10.1016/j.bone.2008.04.013. PMID 18541467.
- ↑ Dalton, Aaron (2005-08-15). Nanotubes May Heal Broken Bones. Wired.
- ↑ Petersen, E. J.; Tu, X.; Dizdaroglu, M.; Zheng, M.; Nelson, B. C. (2013). "Protective Roles of Single-Wall Carbon Nanotubes in Ultrasonication-Induced DNA Base Damage". Small 9 (2): 205. doi:10.1002/smll.201201217. PMID 22987483.
- ↑ Armon Sharei, Janet Zoldan, Andrea Adamo, Woo Young Sim, Nahyun Cho, Emily Jackson, Shirley Mao, Sabine Schneider, Min-Joon Han, Abigail Lytton-Jean, Pamela A. Basto, Siddharth Jhunjhunwala, Jungmin Lee, Daniel A. Heller, Jeon Woong Kang, George C. Hartoularos, Kwang-Soo Kim, Daniel G. Anderson, Robert Langer, and Klavs F. Jensen (2013). "A vector-free microfluidic platform for intracellular delivery". PNAS 110 (6): 2082–2087. Bibcode:2013PNAS..110.2082S. doi:10.1073/pnas.1218705110. PMC 3568376. PMID 23341631.
- ↑ Mogensen, K. B.; Chen, M.; Molhave, K.; Boggild, P.; Kutter, J. R. P. (2011). "Carbon nanotube based separation columns for high electrical field strengths in microchip electrochromatography". Lab on a Chip 11 (12): 2116. doi:10.1039/C0LC00672F.
- ↑ Mogensen, K. B.; Kutter, J. R. P. (2012). "Carbon nanotube based stationary phases for microchip chromatography". Lab on a Chip 12 (11): 1951. doi:10.1039/C2LC40102A.
- ↑ Edwards, Brad C. (2003). The Space Elevator. BC Edwards. ISBN 0-9746517-1-0.
- ↑ Zhang, M.; Fang, S; Zakhidov, AA; Lee, SB; Aliev, AE; Williams, CD; Atkinson, KR; Baughman, RH (2005). "Strong, Transparent, Multifunctional, Carbon Nanotube Sheets". Science 309 (5738): 1215–1219. Bibcode:2005Sci...309.1215Z. doi:10.1126/science.1115311. PMID 16109875.
- ↑ Dalton, Alan B.; Collins, Steve; Muñoz, Edgar; Razal, Joselito M.; Ebron, Von Howard; Ferraris, John P.; Coleman, Jonathan N.; Kim, Bog G.; Baughman, Ray H. (2003). "Super-tough carbon-nanotube fibres". Nature 423 (6941): 703. Bibcode:2003Natur.423..703D. doi:10.1038/423703a. PMID 12802323.
- ↑ Miaudet, P.; Badaire, S.; Maugey, M.; Derré, A.; Pichot, V.; Launois, P.; Poulin, P.; Zakri, C. (2005). "Hot-Drawing of Single and Multiwall Carbon Nanotube Fibers for High Toughness and Alignment". Nano Letters 5 (11): 2212–2215. Bibcode:2005NanoL...5.2212M. doi:10.1021/nl051419w. PMID 16277455.
- ↑ Li, Y.-L.; Kinloch, IA; Windle, AH (2004). "Direct Spinning of Carbon Nanotube Fibers from Chemical Vapor Deposition Synthesis". Science 304 (5668): 276–278. Bibcode:2004Sci...304..276L. doi:10.1126/science.1094982. PMID 15016960.
- ↑ Motta, M.; Moisala, A.; Kinloch, I. A.; Windle, Alan H. (2007). "High Performance Fibres from 'Dog Bone' Carbon Nanotubes". Advanced Materials 19 (21): 3721–3726. doi:10.1002/adma.200700516.
- ↑ Koziol, K.; Vilatela, J.; Moisala, A.; Motta, M.; Cunniff, P.; Sennett, M.; Windle, A. (2007). "High-Performance Carbon Nanotube Fiber". Science 318 (5858): 1892–1895. Bibcode:2007Sci...318.1892K. doi:10.1126/science.1147635. PMID 18006708.
- ↑ Yang, Y.; Chen, X.; Shao, Z.; Zhou, P.; Porter, D.; Knight, D. P.; Vollrath, F. (2005). "Toughness of Spider Silk at High and Low Temperatures". Advanced Materials 17 (1): 84–88. doi:10.1002/adma.200400344.
- ↑ Naraghi, Mohammad; Filleter, Tobin; Moravsky, Alexander; Locascio, Mark; Loutfy, Raouf O.; Espinosa, Horacio D. (2010). "A Multiscale Study of High Performance Double-Walled Nanotube−Polymer Fibers". ACS Nano 4 (11): 6463–6476. doi:10.1021/nn101404u. PMID 20977259.
- ↑ Yildirim, T.; Gülseren, O.; Kılıç, Ç.; Ciraci, S. (2000). "Pressure-induced interlinking of carbon nanotubes". Phys. Rev. B 62 (19): 19. arXiv:cond-mat/0008476. Bibcode:2000PhRvB..6212648Y. doi:10.1103/PhysRevB.62.12648.
- ↑ Postma, Henk W. Ch.; Teepen, T; Yao, Z; Grifoni, M; Dekker, C (2001). "Carbon Nanotube Single-Electron Transistors at Room temperature". Science 293 (5527): 76–9. Bibcode:2001Sci...293...76P. doi:10.1126/science.1061797. PMID 11441175.
- ↑ Collins, Philip G.; Arnold, MS; Avouris, P (2001). "Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown". Science 292 (5517): 706–709. Bibcode:2001Sci...292..706C. doi:10.1126/science.1058782. PMID 11326094.
- ↑ Javey, Ali; Guo, J; Wang, Q; Lundstrom, M; Dai, H (2003). "Ballistic Carbon Nanotube Transistors". Nature 424 (6949): 654–657. Bibcode:2003Natur.424..654J. doi:10.1038/nature01797. PMID 12904787.
- ↑ Javey, Ali; Guo, Jing; Farmer, Damon B.; Wang, Qian; Yenilmez, Erhan; Gordon, Roy G.; Lundstrom, Mark; Dai, Hongjie (2004). "Self-aligned ballistic molecular transistors and electrically parallel nanotube arrays". Nano Letters 4 (7): 1319–1322. arXiv:cond-mat/0406494. Bibcode:2004NanoL...4.1319J. doi:10.1021/nl049222b.
- ↑ Tseng, Yu-Chih; Xuan, Peiqi; Javey, Ali; Malloy, Ryan; Wang, Qian; Bokor, Jeffrey; Dai, Hongjie (2004). "Monolithic Integration of Carbon Nanotube Devices with Silicon MOS Technology". Nano Letters 4 (1): 123–127. Bibcode:2004NanoL...4..123T. doi:10.1021/nl0349707.
- ↑ Gabriel, J. C. P. (2010). "Réseaux 2d aléatoires à nanotubes de carbone". Comptes Rendus Physique 11 (5–6): 362. doi:10.1016/j.crhy.2010.07.016.
- ↑ Gabriel, Jean-Christophe P. (2003). "Large Scale Production of Carbon Nanotube Transistors: A Generic Platforms for Chemical Sensors". Mat. Res. Soc. Symp. Proc. 762: Q.12.7.1.
- ↑ Nanōmix – Breakthrough Detection Solutions with the Nanoelectronic Sensation Technology. Nano.com.
- ↑ Gabriel, Jean-Christophe P. "Dispersed Growth Of Nanotubes on a substrate". Patent WO 2004040671A2.
- ↑ Bradley, Keith; Gabriel, Jean-Christophe P.; Grüner, George (2003). "Flexible nanotube transistors". Nano Letters 3 (10): 1353–1355. Bibcode:2003NanoL...3.1353B. doi:10.1021/nl0344864.
- ↑ Armitage, Peter N.; Bradley, Keith; Gabriel, Jean-Christophe P.; Gruner, George. "Flexible nanostructure electronic devices". United States Patent US8456074.
- ↑ Kordás, K.; TóTh, G.; Moilanen, P.; KumpumäKi, M.; VäHäKangas, J.; UusimäKi, A.; Vajtai, R.; Ajayan, P. M. (2007). "Chip cooling with integrated carbon nanotube microfin architectures". Appl. Phys. Lett. 90 (12): 123105. Bibcode:2007ApPhL..90l3105K. doi:10.1063/1.2714281.
- ↑ Lee, Robert. (2002-10-03) Scientists Build First Nanotube Computer – WSJ.com. The Wall Street Journal.
- ↑ Hsu, Jeremy. (2013-09-24) Carbon Nanotube Computer Hints at Future Beyond Silicon Semiconductors. Scientific American.
- ↑ BBC News – First computer made of carbon nanotubes is unveiled. BBC.
- ↑ Janas, Dawid; Herman, Artur P.; Boncel, Slawomir; Koziol, Krzysztof K. (February 22, 2014). "Iodine monochloride as a powerful enhancer of electrical conductivity of carbon nanotube wires". Carbon 73: 225–233. doi:10.1016/j.carbon.2014.02.058.
- ↑ "Nanocables light way to the future". YouTube. September 9, 2011.
- ↑ Zhao, Yao; Wei, Jinquan; Vajtai, Robert; Ajayan, Pulickel M.; Barrera, Enrique V. (September 6, 2011). "Iodine doped carbon nanotube cables exceeding specific electrical conductivity of metals". Scientific Reports (Nature) 1: 83. Bibcode:2011NatSR...1E..83Z. doi:10.1038/srep00083.
- ↑ Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D. N.; Yumura, M.; Hata, K. (2013). "One hundred fold increase in current carrying capacity in a carbon nanotube–copper composite". Nature Communications 4. doi:10.1038/ncomms3202.
- ↑ "Beyond Batteries: Storing Power in a Sheet of Paper". Eurekalert.org. August 13, 2007.
- ↑ "New Flexible Plastic Solar Panels Are Inexpensive And Easy To Make". ScienceDaily. July 19, 2007.
- ↑ Guldi, Dirk M., G.M.A. Rahman, Maurizio Prato, Norbert Jux, Shubui Qin, and Warren Ford (2005). "Single-Wall Carbon Nanotubes as Integrative Building Blocks for Solar-Energy Conversion". Angewandte Chemie 117 (13): 2051–2054. doi:10.1002/ange.200462416. PMID 15724261.
- ↑ Dillon, A. C.; Jones, K. M.; Bekkedahl, T. A.; Kiang, C. H.; Bethune, D. S.; Heben, M. J. (1997). "Storage of hydrogen in single-walled carbon nanotubes". Nature 386 (6623): 377. doi:10.1038/386377a0.
- ↑ Jhi, S. H.; Kwon, Y. K.; Bradley, K.; Gabriel, J. C. P. (2004). "Hydrogen storage by physisorption: Beyond carbon". Solid State Communications 129 (12): 769. doi:10.1016/j.ssc.2003.12.032.
- ↑ Safa, S., Mojtahedzadeh Larijani, M., Fathollahi, V., Kakuee, O. R. (2010). "Investigating Hydrogen Storage Behavior of Carbon Nanotubes at Ambient Temperature and Above by Ion Beam Analysis". NANO 5 (6): 341–347. doi:10.1142/S1793292010002256.
- ↑ Barghi, S. H.; Tsotsis, T. T.; Sahimi, M. (2014). "Chemisorption, physisorption and hysteresis during hydrogen storage in carbon nanotubes". International Journal of Hydrogen Energy 39 (3): 1390. doi:10.1016/j.ijhydene.2013.10.163.
- ↑ Yuca, N., Karatepe, N. (2011). "Hydrogen Storage in Single-Walled Carbon Nanotubes Purified by Microwave Digestion Method". World Academy of Science, Engineering and Technology 79: 605–610.
- ↑ Halber, Deborah. MIT LEES on Batteries. Lees.mit.edu.
- ↑ Bourzac, Katherine. "Nano Paint Could Make Airplanes Invisible to Radar." Technology Review. MIT, 5 December 2011.
- ↑ Pötschke, P.; Andres, T.; Villmow, T.; Pegel, S.; Brünig, H.; Kobashi, K.; Fischer, D.; Häussler, L. (2010). "Liquid sensing properties of fibres prepared by melt spinning from poly(lactic acid) containing multi-walled carbon nanotubes". Composites Science and Technology 70 (2): 343. doi:10.1016/j.compscitech.2009.11.005.
- ↑ Chen, P.; Kim, H. S.; Kwon, S. M.; Yun, Y. S.; Jin, H. J. (2009). "Regenerated bacterial cellulose/multi-walled carbon nanotubes composite fibers prepared by wet-spinning". Current Applied Physics 9 (2): e96. doi:10.1016/j.cap.2008.12.038.
- ↑ Coleman, J. N.; Khan, U.; Blau, W. J.; Gun’Ko, Y. K. (2006). "Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites". Carbon 44 (9): 1624. doi:10.1016/j.carbon.2006.02.038.
- ↑ Shim, B. S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N. A. (2008). "Smart Electronic Yarns and Wearable Fabrics for Human Biomonitoring made by Carbon Nanotube Coating with Polyelectrolytes". Nano Letters 8 (12): 4151–7. doi:10.1021/nl801495p. PMID 19367926.
- ↑ Panhuis, M. I. H.; Wu, J.; Ashraf, S. A.; Wallace, G. G. (2007). "Conducting textiles from single-walled carbon nanotubes". Synthetic Metals 157 (8–9): 358. doi:10.1016/j.synthmet.2007.04.010.
- ↑ Hu, L.; Pasta, M.; Mantia, F. L.; Cui, L.; Jeong, S.; Deshazer, H. D.; Choi, J. W.; Han, S. M.; Cui, Y. (2010). "Stretchable, Porous, and Conductive Energy Textiles". Nano Letters 10 (2): 708–14. doi:10.1021/nl903949m. PMID 20050691.
- ↑ X Li, T Gu, B Wei; Gu; Wei (2012). "Dynamic and Galvanic Stability of Stretchable Supercapacitors". Nano letters 12 (12): 6366–6371. doi:10.1021/nl303631e. PMID 23167804.
- ↑ F. Alimohammadi, M. Parvinzadeh, A. Shamei (2011) "Carbon Nanotube Embedded Textiles", U.S. Patent 0,171,413.
- ↑ Alimohammadi, F.; Parvinzadeh Gashti, M.; Shamei, A. (2012). "Functional cellulose fibers via polycarboxylic acid/carbon nanotube composite coating". Journal of Coatings Technology and Research 10: 123. doi:10.1007/s11998-012-9429-3.
- ↑ Alimohammadi, F.; Gashti, M. P.; Shamei, A. (2012). "A novel method for coating of carbon nanotube on cellulose fiber using 1,2,3,4-butanetetracarboxylic acid as a cross-linking agent". Progress in Organic Coatings 74 (3): 470. doi:10.1016/j.porgcoat.2012.01.012.
- ↑ "Super-nanotubes: ‘remarkable’ spray-on coating combines carbon nanotubes with ceramic". KurzweilAI.
- ↑ Bhandavat, R.; Feldman, A.; Cromer, C.; Lehman, J.; Singh, G. (2013). "Very High Laser-Damage Threshold of Polymer-derived Si(B)CN- Carbon Nanotube Composite Coatings". ACS Applied Materials & Interfaces 5 (7): 2354. doi:10.1021/am302755x.
- ↑ Lin Xiao, Lin; Zhuo Chen; Chen Feng; Liang Liu; Zai-Qiao Bai; Yang Wang; Li Qian; Yuying Zhang; Qunqing Li; Kaili Jiang; Shoushan Fan (2008). "Flexible, Stretchable, Transparent Carbon Nanotube Thin Film Loudspeakers". Nano Letters 8 (12): 4539–4545. Bibcode:2008NanoL...8.4539X. doi:10.1021/nl802750z. PMID 19367976.
- ↑ Hot nanotube sheets produce music on demand, New Scientists News, 31 October 2008
- ↑ Yang Wei, Yang; Xiaoyang Lin; Kaili Jiang; Peng Liu; Qunqing Li; Shoushan Fan (2013). "Thermoacoustic Chips with Carbon Nanotube Thin Yarn Arrays". Nano Letters 13 (10): 4795–801. Bibcode:2013NanoL..13.4795W. doi:10.1021/nl402408j. PMID 24041369.
- ↑
- ↑ Camilli, L.; Pisani, C.; Gautron, E.; Scarselli, M.; Castrucci, P.; d'Orazio, F.; Passacantando, M.; Moscone, D.; De Crescenzi, M. (2014). "A three-dimensional carbon nanotube network for water treatment". Nanotechnology 25 (6): 065701. doi:10.1088/0957-4484/25/6/065701. PMID 24434944.
- ↑ Quick, Darren (17 April 2012) Reusable oil-absorbing nanosponges could soak up oil spills. Gizmag
- ↑ Hashim, D. P.; Narayanan, N. T.; Romo-Herrera, J. M.; Cullen, D. A.; Hahm, M. G.; Lezzi, P.; Suttle, J. R.; Kelkhoff, D.; Muñoz-Sandoval, E.; Ganguli, S.; Roy, A. K.; Smith, D. J.; Vajtai, R.; Sumpter, B. G.; Meunier, V.; Terrones, H.; Terrones, M.; Ajayan, P. M. (2012). "Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions". Scientific Reports 2. doi:10.1038/srep00363.
- ↑ Zhang, S.J.; Shao, T.; Kose, H.S.; Karanfil, T. (2010). "Adsorption of Aromatic Compounds by Carbonaceous Adsorbents: A Comparative Study on Granular Activated Carbon, Activated Carbon Fiber, and Carbon Nanotubes". Environmental Science and Technology 44 (16): 6377–6383. doi:10.1021/es100874y.
- ↑ Apul, O.G.; Wang, Q.; Zhou, Y.; Karanfil, T. (2013). "Adsorption of aromatic organic contaminants by graphene nanosheets: Comparison with carbon nanotubes and activated carbon". Water Research 47 (4): 1648–1654. doi:10.1016/j.watres.2012.12.031.
- ↑ Apul, O.; Karanfil, T. (2015). "Adsorption of Synthetic Organic Contaminants by Carbon Nanotubes: A Critical Review". Water Research 68: 34–55. doi:10.1016/j.watres.2014.09.032.
- ↑ Zhang, S.J.; Shao, T.; Karanfil, T. (2010). "The Effects of Dissolved Natural Organic Matter on the Adsorption of Synthetic Organic Chemicals by Activated Carbons and Carbon Nanotubes". Water Research 45 (3): 1378–1386. doi:10.1016/j.watres.2010.10.023.
- ↑ Fasano, Matteo; Chiavazzo, Eliodoro; Asinari, Pietro (2014). "Water transport control in carbon nanotube arrays". Nanoscale Research Letters 9 (1): 559. doi:10.1186/1556-276X-9-559.
- ↑ Polikarpova, N.P.; Zaporotskova, I. V.; Vilkeeva, D.E.; Polikarpov, D. I. (2014). "Sensor properties of carboxyl-modified carbon nanotubes" (PDF). Nanosystems: physics, chemistry, mathematics. 5 (1).
- ↑ Simmons, Trevor; Hashim, D; Vajtai, R; Ajayan, PM (2007). "Large Area-Aligned Arrays from Direct Deposition of Single-Wall Carbon Nanotubes". J. Am. Chem. Soc. 129 (33): 10088–10089. doi:10.1021/ja073745e. PMID 17663555.
- ↑ Matson, Michael L; Wilson, Lon J (2010). "Nanotechnology and MRI contrast enhancement". Future Medicinal Chemistry 2 (3): 491–502. doi:10.4155/fmc.10.3. PMID 21426177.
- ↑ Zhang, J.; Liu, X.; Blume, R.; Zhang, A.; Schlögl, R.; Su, D. S. (2008). "Surface-Modified Carbon Nanotubes Catalyze Oxidative Dehydrogenation of n-Butane". Science 322 (5898): 73–77. Bibcode:2008Sci...322...73Z. doi:10.1126/science.1161916. PMID 18832641.
- ↑ Frank, B.; Blume, R.; Rinaldi, A.; Trunschke, A.; Schlögl, R. (2011). "Oxygen Insertion Catalysis by sp2 Carbon". Angew. Chem. Int. Ed. 50 (43): 10226–10230. doi:10.1002/anie.201103340.
- ↑ Halford, Bethany (9 February 2009). "Nanotube Catalysts". Chemical & Engineering News 87 (6): 7. doi:10.1021/cen-v087n006.p007a.
- ↑ Dooley, Erin E. (February 2013). "The Beat, A New Lighting Alternative?". Environmental Health Perspectives (National Institute of Environmental Health Sciences (NIEHS)) 121 (2): A47. doi:10.1289/ehp.121-a47.
- ↑ Di Giacomo, R.; Maresca, B.; Porta, A.; Sabatino, P.; Carapella, G.; Neitzert, H. C. (2013). "Candida albicans/MWCNTs: A Stable Conductive Bio-Nanocomposite and Its Temperature-Sensing Properties". IEEE Transactions on Nanotechnology 12 (2): 111. doi:10.1109/TNANO.2013.2239308.
- ↑ "Speciality Chemicals Magazine - March 2015 digital edition". Retrieved 2015-04-16.
- ↑ Xu, Ming; Futaba, Don N.; Yamada, Takeo; Yumura, Motoo; Hata, Kenji (2010). "Carbon Nanotubes with Temperature-Invariant Viscoelasticity from –196° to 1000°C". Science (in English) 330 (6009): 1364–1368. doi:10.1126/science.1194865. ISSN 0036-8075. PMID 21127248. Retrieved 2015-04-16.
- ↑ 227.0 227.1 Monthioux, Marc; Kuznetsov, V (2006). "Who should be given the credit for the discovery of carbon nanotubes?" (PDF). Carbon 44 (9): 1621–1623. doi:10.1016/j.carbon.2006.03.019.
- ↑ Oberlin, A.; Endo, M.; Koyama, T. (1976). "Filamentous growth of carbon through benzene decomposition". Journal of Crystal Growth 32 (3): 335–349. Bibcode:1976JCrGr..32..335O. doi:10.1016/0022-0248(76)90115-9.
- ↑ Endo, Morinobu; Dresselhaus, M. S. (October 26, 2002). "Carbon Fibers and Carbon Nanotubes (Interview, Nagano, Japan)" (PDF).
- ↑ Abrahamson, John; Wiles, Peter G.; Rhoades, Brian L. (1999). "Structure of Carbon Fibers Found on Carbon Arc Anodes". Carbon 37 (11): 1873–1874. doi:10.1016/S0008-6223(99)00199-2.
- ↑ Izvestiya Akademii Nauk SSSR, Metals. 1982, #3, pp.12–17 (in Russian)
- ↑ US 4663230, Tennent, Howard G., "Carbon fibrils, method for producing same and compositions containing same", issued 1987-05-05
- ↑ Iijima, Sumio (7 November 1991). "Helical microtubules of graphitic carbon". Nature 354 (6348): 56–58. Bibcode:1991Natur.354...56I. doi:10.1038/354056a0.
- ↑ Mintmire, J.W.; Dunlap, BI; White, CT (1992). "Are Fullerene Tubules Metallic?". Phys. Rev. Lett. 68 (5): 631–634. Bibcode:1992PhRvL..68..631M. doi:10.1103/PhysRevLett.68.631. PMID 10045950.
- ↑ Bethune, D. S.; Kiang, C. H.; De Vries, M. S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. (1993). "Cobalt-catalyzed growth of carbon nanotubes with single-atomic-layer walls". Nature 363 (6430): 605–607. Bibcode:1993Natur.363..605B. doi:10.1038/363605a0.
- ↑ Iijima, Sumio; Ichihashi, Toshinari (1993). "Single-shell carbon nanotubes of 1-nm diameter". Nature 363 (6430): 603–605. Bibcode:1993Natur.363..603I. doi:10.1038/363603a0.
- ↑ "The Discovery of Single-Wall Carbon Nanotubes at IBM". IBM.
- ↑ 238.0 238.1 Krätschmer, W.; Lamb, Lowell D.; Fostiropoulos, K.; Huffman, Donald R. (1990). "Solid C60: a new form of carbon". Nature 347 (6291): 354–358. Bibcode:1990Natur.347..354K. doi:10.1038/347354a0.
- ↑ Kroto, H. W.; Heath, J. R.; O'Brien, S. C.; Curl, R. F.; Smalley, R. E. (1985). "C60: Buckminsterfullerene". Nature 318 (6042): 162–163. Bibcode:1985Natur.318..162K. doi:10.1038/318162a0.
External links
| Wikimedia Commons has media related to Carbon nanotube. |
- Nanohedron.com image gallery with carbon nanotubes
- The stuff of dreams, CNET
- The Nanotube site. Last updated 2009.05.03
- EU Marie Curie Network CARBIO: Multifunctional carbon nanotubes for biomedical applications
- Carbon nanotube on arxiv.org
- C60 and Carbon Nanotubes a short video explaining how nanotubes can be made from modified graphite sheets and the three different types of nanotubes that are formed
- Carbon Nanotubes & Buckyballs.
- The Wondrous World of Carbon Nanotubes
- Learning module for Bandstructure of Carbon Nanotubes and Nanoribbons
- Durability of carbon nanotubes and their potential to cause inflammation by Dr Megan Osmond and others. (SafeWork Australia, May 2011). This was a collaboration between the Institute of Occupational Medicine, Edinburgh University and CSIRO in Australia.
- NT06 Seventh International Conference on the Science and Application of Nanotubes
- NT05 Sixth International Conference on the Science and Application of Nanotubes
- Selection of free-download articles on carbon nanotubes
- First computer made of carbon nanotubes is unveiled (BBC News) 2013-09-25
- Research using carbon nanotubes for microfabrication and in other applications
- Nanotech drug smugglers
| ||||||||||||||||||||||||||||||