Cancer immunotherapy
Cancer immunotherapy is the use of the immune system to treat cancer. Immunotherapies fall into three main groups: cellular, antibody and cytokine. They exploit the fact that cancer cells often have subtly different molecules on their surface that can be detected by the immune system. These molecules, known as cancer antigens, are most commonly proteins but also include molecules such as carbohydrates. Immunotherapy is used to provoke the immune system into attacking the tumor cells by using these antigens as targets.
Cellular therapies, also known as cancer vaccines, usually involve the removal of immune cells from someone with cancer, either from the blood or from a tumor. Immune cells specific for the tumor are activated, cultured and returned to the patient where the immune cells attack the cancer. Cell types that can be used in this way are natural killer cells, lymphokine-activated killer cells, cytotoxic T cells and dendritic cells. The only cell-based therapy currently approved for use is Dendreon's Provenge, which is used for the treatment of prostate cancer.
Antibody therapies are the most successful immunotherapy, with approved treatments for a wide range of cancers. Antibodies are proteins produced by the immune system that bind to a target antigen on the cell surface. In normal physiology the immune system uses them to fight pathogens. Each antibody is specific to one or a few proteins and those that bind to cancer antigens are used in the treatment of cancer. Cell surface receptors are common targets for antibody therapies and include the epidermal growth factor receptor and HER2. Once bound to a cancer antigen, antibodies can induce antibody-dependent cell-mediated cytotoxicity, activate the complement system, prevent a receptor from interacting with its ligand or deliver a payload of chemotherapy or radiation, all of which can lead to cell death. Thirteen antibodies are approved for the treatment of cancer: Alemtuzumab, Bevacizumab, Brentuximab vedotin, Cetuximab, Gemtuzumab ozogamicin, Ibritumomab tiuxetan, Ipilimumab, Nivolumab, Ofatumumab, Panitumumab, Rituximab, Tositumomab and Trastuzumab.
Interleukin-2 and interferon-α are examples of cytokines, proteins that regulate and coordinate the behaviour of the immune system. They have the ability to enhance anti-tumor activity and thus can be used as cancer treatments. Interferon-α is used in the treatment of hairy-cell leukaemia, AIDS-related Kaposi's sarcoma, follicular lymphoma, chronic myeloid leukaemia and malignant melanoma. Interleukin-2 is used in the treatment of malignant melanoma and renal cell carcinoma.
History
Cancer immunotherapy arose from advances in oncology and immunology. Immunotherapy began in 1796 when Edward Jenner produced the first vaccine involving immunisation with cowpox to prevent smallpox. Towards the end of the 19th century Emil von Behring and Shibasaburō Kitasato discovered that injecting animals with diphtheria toxin produced blood serum with antitoxins to it.
Paul Ehrlich's research gave rise to the "magic bullet" concept; using antibodies to specifically target a disease. The production of pure monoclonal antibodies for therapeutic use became available in 1975 when Georges J. F. Köhler and Cesar Milstein produced the hybridoma technology. In 1997 Rituximab, the first antibody treatment for cancer, was approved by the FDA for treatment of follicular lymphoma. Since this approval, 11 other antibodies have been approved for cancer; Trastuzumab (1998), Gemtuzumab ozogamicin (2000), Alemtuzumab (2001), Ibritumomab tiuxetan (2002), Tositumomab (2003), Cetuximab (2004), Bevacizumab (2004), Panitumumab (2006), Ofatumumab (2009), Ipilimumab (2011) and Brentuximab vedotin (2011).
The production of vaccines for cancer came later than the use of monoclonal antibodies. The first cell-based immunotherapy cancer vaccine, Sipuleucel-T, was approved in 2010 for the treatment of prostate cancer.[1][2]
Cellular immunotherapy
Dendritic cell therapy

Dendritic cell therapy provokes anti-tumor responses by causing dendritic cells to present tumor antigens. Dendritic cells present antigens to lymphocytes, which activates them, priming them to kill other cells that present the antigen. In cancer treatment they aid cancer antigen targeting.[3] The only approved cellular therapy for cancer is Sipuleucel-T.
One method of inducing dendritic cells to present tumor antigens is by vaccination with short peptides (small parts of protein that correspond to the protein antigens on cancer cells). These peptides on their own do not stimulate a strong immune response and may be given in combination with adjuvants (highly immunogenic substances). This provokes a strong response, while also producing a (sometimes) robust anti-tumor response by the immune system. Other adjuvants include proteins or other chemicals that attract and/or activate dendritic cells, such as granulocyte macrophage colony-stimulating factor (GM-CSF). Dendritic cells can also be activated within the body (in vivo) by making tumour cells express (GM-CSF). This can be achieved by either genetically engineering tumor cells that produce GM-CSF or by infecting tumor cells with an oncolytic virus that expresses GM-CSF.
Another strategy is to remove dendritic cells from the blood of a patient and activate them outside the body (ex vivo). The dendritic cells are activated in the presence of tumor antigens, which may be a single tumor-specific peptide/protein or a tumor cell lysate (a solution of broken down tumor cells). These activated dendritic cells are put back into the body where they provoke an immune response to the cancer cells. Adjuvants are sometimes used systemically to increase the anti-tumor response provided by ex vivo activated dendritic cells. More modern dendritic cell therapies include the use of antibodies that bind to receptors on the surface of dendritic cells. Antigens can be added to the antibody and can induce the dendritic cells to mature and provide immunity to the tumor. Dendritic cell receptors such as TLR3, TLR7, TLR8 or CD40 have been used as targets by antibodies to produce immune responses.[3]
Sipuleucel-T
Sipuleucel-T (Provenge) is the first approved cancer vaccine. It was approved for treatment of asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer in 2010. The treatment consists of removal of antigen-presenting cells from blood by leukapheresis, and growing them with the fusion protein PA2024 made from GM-CSF and prostatic acid phosphatase (PAP). These cells are infused back into the patient to induce an immune response because PAP protein is prostate specific. This process is repeated three times.[4][5][6][7]
Antibody therapy

Antibodies are a key component of the adaptive immune response, playing a central role in both in the recognition of foreign antigens and the stimulation of an immune response to them. It is not surprising therefore, that many immunotherapeutic approaches involve the use of antibodies. The advent of monoclonal antibody technology has made it possible to raise antibodies against specific antigens, such as the unusual antigens present on tumor surfaces.
Types of monoclonal antibodies
Two types of monoclonal antibodies are used in cancer treatments:[8]
- Naked monoclonal antibodies are antibodies without modification. Most of the currently used antibodies therapies are naked.
- Conjugated monoclonal antibodies are joined to another molecule, which is either toxic to cells or radioactive. The toxic chemicals are those typically used as chemotherapy drugs, but other toxins can be used. The antibody binds to specific antigens on cancer cell surfaces, directing the therapy to the tumor. Radioactive compound-linked antibodies are referred to as radiolabelled. If the antibodies are labelled with chemotherapy or toxins, they are known as chemolabelled or immunotoxins, respectively.
Antibodies are also referred to as murine, chimeric, humanized and human. Murine antibodies were the first to be produced, and carry a great risk of immune reaction, because the antibodies are from a different species. Chimeric antibodies were the first attempt to reduce the immunogenicity of these antibodies. They are murine antibodies with a specific part of the antibody replaced with the corresponding human counterpart, known as the constant region. Humanized antibodies are almost completely human; only the complementarity determining regions of the variable regions are derived from murine antibodies. Human antibodies have completely human DNA.[9]
Cell death mechanisms
Antibody-dependent cell-mediated cytotoxicity (ADCC)
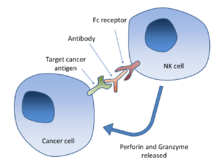
aAntibody-dependent cell-mediated cytotoxicity (ADCC) is a mechanism of attack by the immune system that requires antibodies to bind to target cell surfaces. Antibodies are formed of a binding region (Fab) and the Fc region that can be detected by immune cells via their Fc surface receptors. Fc receptors are found on many immune system cells, including natural killer cells. When natural killer cells encounter antibody-coated cells, the latter's Fc regions interact with their Fc receptors, leading to the release of perforin and granzyme B. These two chemicals programmed cell death (apoptosis) in the tumor cell. Effective antibodies include Rituximab, Ofatumumab, Trastuzumab, Cetuximab and Alemtuzumab. Third generation antibodies under development have altered Fc regions that have higher affinity for a specific type of Fc receptor, FcγRIIIA, which can dramatically increase ADCC.[10][11]
Complement
The complement system includes blood proteins that can cause cell death after an antibody binds to the cell surface (this is the classical complement pathway, among the ways of complement activation). Generally the system deals with foreign pathogens, but can be activated with therapeutic antibodies in cancer. The system can be triggered if the antibody is chimeric, humanized or human; as long as it contains the IgG1 Fc region. Complement can lead to cell death by activation of the membrane attack complex, known as complement-dependent cytotoxicity; enhancement of antibody-dependent cell-mediated cytotoxicity; and CR3-dependent cellular cytotoxicity. Complement-dependent cytotoxicity occurs when antibodies bind to the cancer cell surface, the C1 complex binds to these antibodies and subsequently protein pores are formed in the cancer cell membrane.[12]
Cell signalling

Antibodies that bind to molecules on cancer cells or bind to molecules in the blood can affect cell signalling in various ways. The antibodies can bind to a receptor and prevent binding with external proteins, peptides or small molecules that would normally bind to the receptor (called ligands). Cetuximab and Trastuzumab target growth factor receptors. Antibodies can bind the ligands themselves, such as vascular endothelial growth factor (VEGF), which is involved in blood vessel formation. Bevacizumab is a clinically used antibody that binds VEGF. These receptor-ligand interactions may be essential for cancer cell survival, so blocking them can induce their death. Such antibodies are known as antagonists, but antibodies can also activate signalling by binding to receptors, in which case they are known as agonists. One signalling pathway that is activated by antibodies is the apoptotic pathway.[8]
Payload
Conjugated antibodies carry a payload that is either a drug (usually a chemotherapeutic), toxin, small interfering RNA or radioisotope. Radioimmunotherapy is the term for the use of antibodies conjugated to a radioisotope against cellular antigens. Most research involves their application to lymphomas, as these are highly radio-sensitive malignancies.[13] Out of the 12 approved antibodies used in cancer, two use toxic compounds (Gemtuzumab ozogamicin - calicheamicin and Brentuximab vedotin - monomethyl auristatin E) and two are radiolabelled (Tositumomab - 131I and Ibritumomab tiuxetan - 90Y). These antibodies specifically bind to their targets on the surface of cancer cells and the payloads they are attached to lead to cancer cell death.[8]
Approved antibodies
| Antibody | Brand name | Type | Target | Approval date | Approved treatment(s) |
|---|---|---|---|---|---|
| Alemtuzumab | Campath | humanized | CD52 | 2001 | B-cell Chronic lymphocytic leukemia (CLL)[15] |
| Bevacizumab | Avastin | humanized | vascular endothelial growth factor | 2004 | metastatic colorectal cancer [16] |
| 2006 | non-small cell lung cancer[17] | ||||
| 2009 | renal cell carcinoma[18] | ||||
| 2009 | glioblastoma multiforme[19] | ||||
| Brentuximab vedotin | Adcetris | chimeric | CD30 | 2011 | relapsed Hodgkin lymphoma[20] |
| 2011 | relapsed Anaplastic large-cell lymphoma[20] | ||||
| Cetuximab | Erbitux | chimeric | epidermal growth factor receptor | 2004 | colorectal cancer[21] |
| 2006 | advanced squamous cell carcinoma of the head and neck (SCCHN)[21] | ||||
| 2011 | recurrent locoregional or metastatic squamous cell head and neck cancer[22] | ||||
| 2012 | EGFR-expressing metastatic colorectal cancer[21] | ||||
| Gemtuzumab ozogamicin | Mylotarg | humanized | CD33 | 2000 | acute myelogenous leukemia (with calicheamicin)[23] |
| Ibritumomab tiuxetan | Zevalin | murine | CD20 | 2002 | non-Hodgkin lymphoma (with yttrium-90)[24] |
| Ipilimumab | Yervoy | human | CTLA4 | 2011 | metastatic melanoma[25] |
| Ofatumumab | Arzerra | human | CD20 | 2009 | refractory CLL[26] |
| Panitumumab | Vectibix | human | epidermal growth factor receptor | 2006 | metastatic colorectal cancer[27] |
| Rituximab | Rituxan, Mabthera | chimeric | CD20 | 1997 | non-Hodgkin lymphoma[28] |
| 2010 | CLL[29] | ||||
| Tositumomab | Bexxar | murine | CD20 | 2003 | Non-Hodgkin lymphoma[30] |
| Trastuzumab | Herceptin | humanized | ErbB2 | 1998 | breast cancer[31] |
Alemtuzumab
Alemtuzumab (Campeth-1H) is an anti-CD52 humanized IgG1 monoclonal antibody indicated for the treatment of fludarabine-refractory chronic lymphocytic leukemia (CLL), cutaneous T-cell lymphoma, peripheral T-cell lymphoma and T-cell prolymphocytic leukemia. CD52 is found on >95% of peripheral blood lymphocytes (both T-cells and B-cells) and monocytes, but its function in lymphocytes is unknown. Upon binding to CD52, alemtuzumab initiates its cytotoxic effect by complement fixation and antibody-dependent cell-mediated cytotoxicity mechanisms. Due to the antibody target (cells of the immune system) common complications of alemtuzumab therapy are infection, toxicity and myelosuppression.[32][33][34][35]
Bevacizumab
Bevacizumab (Avastin) is a humanized IgG1 monoclonal antibody that binds to vascular endothelial growth factor-A (VEGF-A), referred to commonly as VEGF without a suffix. Normally VEGF binds to the VEGF-receptor, activating signalling pathways within blood vessel endothelial cells. A marked increase in VEGF expression within the tumor environment stimulates angiogenesis (blood vessel production), which is essential for tumor growth. These blood vessels, however, are not well-formed and lead to poor blood flow, which also affects drug delivery to cancer cells.[36][37][38]
Bevacizumab binds to and physically blocks VEGF, preventing receptor activation, known as steric interference. Bevacizumab's action on VEGF has three possible effects on tumor vasculature: it may cause microvessels to regress; it can normalise tumor blood vessels, allowing better delivery of other drugs; and it can halt vascularization. Normalisation of faulty vessels may be why Bevacizumab is particularly effective in combination with conventional drugs.[37][38][39]
Bevacizumab is licensed for colon cancer, kidney cancer, lung cancer, ovarian cancer, glioblastoma and/or breast cancer, in various countries. Bevacizumab increases survival, progression-free survival, response rate and response duration, but its mechanism of action does not cure them.[38][39][40]
Brentuximab vedotin
Brentuximab vedotin is a second generation chimeric IgG1 antibody drug conjugate used for Hodgkin lymphoma and anaplastic large cell lymphoma (ALCL). It is an antibody conjugated to monomethyl auristatin E, a drug that prevents cell division by disrupting microtubules. The antibody binds to CD30, often found highly expressed on the surface of Hodgkin lymphoma and ALCL cells, and then cross the cell membrane where the drug detaches from the antibody and exerts its cellular effects. By preventing cell division, it kills cancer cells by inducing apoptosis.[41][42]
Cetuximab
Cetuximab (Erbitux) is a chimeric IgG1 monoclonal antibody that targets the extracellular domain (part of the receptor outside the cell) of the epidermal growth factor receptor (EGFR). It is used for colorectal cancer and head and neck cancer. Once a ligand binds to the cell, signalling pathways are activated inside the cell that are associated with malignant characteristics. These include the PI3K/AKT and KRAS/BRAF/MEK/ERK pathways that cause cancer cell proliferation, invasion, differentiation and cancer stem cell renewal.[35][40][43]
Cetuximab functions by competitively inhibiting ligand binding, thereby preventing EGFR activation. It also induces ADCC and leads to increased levels Bax levels, which activates apoptosis. KRAS, a down-stream protein of EGFR, may be mutated in some cases of cancer and remains constitutively active, irrespective of EGFR blocking. Cetuximab is only effective in the treatment of colorectal cancers with wild-type (unmutated) KRAS genes, approximately 40% of cases.[35][40]
Gemtuzumab ozogamicin
Gemtuzumab ozogamicin is an “immuno-conjugate” of an IgG4 anti-CD33 antibody chemically linked to a cytotoxic calicheamicin derivative.[44] It was used for acute myeloid leukaemia (AML) after accelerated approval by the Food and Drug Administration in May 2000. In June 2010 it was withdrawn over safety concerns.[45] Further research and clinical trials indicated that it might be safe and effective in a subset of cases with favourable prognoses.[46]
The antibody binds to the CD33 antigen, which is found on the surface of myeloblasts (immature precursor cells) in AML in approximately 80% of cases. The antibody is similar to a chemical derivative of calicheamicin, (N-acetyl-γ calicheamicin 1,2-dimethyl hydrazine dichloride) that is highly toxic to cells due to its ability to bind to DNA. Because the antibody is an IgG4 isotype, it is internalised into the cancer cells and does not activate antibody-dependent cell-mediated cytotoxicity or complement-mediated cytotoxicity. Inside intracellular lysozomes, the pH is very acidic (approximately pH 4) causing the release of calicheamicin from the antibody. Once released it is activated and free to bind to DNA, which leads to breakage of DNA and subsequent cell death.[44]
Ibritumomab tiuxetan

Ibritumomab tiuxetan (Zevalin) is a murine anti-CD20 antibody chemically linked to a chelating agent that binds the radioisotope yttrium-90 (90Y). It is used to treat a specific type of non-Hodgkin lymphoma, follicular lymphoma, a B-cell tumor. The antibody target, CD20, is primarily expressed on the surface of B-cells, which allows the 90Y to emit a targeted dose of beta radiation to the tumor. 90Y has a half-life of 64 hours (2.67 days) and a tissue penetration of 1-5 millimetres (90% of its energy is absorbed within a 5.3mm sphere). Ibritumomab tiuxetan and the radioisotope are obtained separately and mixed shortly before administration. The tiuxetan chelating agent attached to the antibody binds the radioisotope and forms the active drug.[47][48]
Ipilimumab
Ipilimumab (Yervoy) is a human IgG1 antibody that binds the surface protein CTLA4. In normal physiology T-cells are activated by two signals: the T-cell receptor binding to and antigen-MHC complex and T-cell surface receptor CD28 binding to CD80 or CD86 on the surface of antigen presenting cells. CTLA4 binds to CD80 or CD86, preventing the binding of CD28 to these surface proteins and therefore negatively regulating the activation of T-cells.[49][50][51][52]
Active cytotoxic T-cells are required for the immune system to attack melanoma cells. Active melanoma-specific cytotoxic T-cells that would normally be inhibited can produce an effective anti-tumor response. Also, ipilumumab can cause a shift in the ratio of regulatory T-cells to cytotoxic T-cells. Regulatory T-cells inhibit other T-cells, which may benefit the tumor. Increasing the amount of cytotoxic T-cells and decreasing the regulatory T-cells is another mechanism by which ipilumumab increases the anti-tumor response.[49][50][51][52]
Nimotuzumab
Nimotuzumab is a chimeric human-mouse anti-EGFR monoclonal antibody invented in Cuba that has been developed by several companies.[53] It has been approved for squamous cell carcinoma in head and neck (SCCHN) in India, Cuba, Argentina, Colombia, Ivory Coast, Gabon, Ukraine, Peru and Sri Lanka; for glioma (pediatric and adult) in Cuba, Argentina, Philippines and Ukraine; for nasopharyngeal cancer in China and has been granted orphan drug status for glioma in USA and for glioma and pancreatic cancer in Europe.[54] It was in Phase I and II clinical trials in other indications as of 2014.[55]
Ofatumumab
Ofatumumab is a second generation human IgG1 antibody that binds to CD20. It is used in the treatment of chronic lymphocytic leukemia (CLL) because the cancerous cells of CLL are usually CD20-expressing B-cells. Unlike Rituximab, which binds to a large loop of the CD20 protein, Ofatumumab binds to a separate small loop. This may be the reason for the two drugs' different characteristics. Compared to Rituximab, Ofatumumab induces complement-dependent cytotoxicity at a lower dose with less immunogenicity.[56][57]
Panitumumab
Panitumumab (Vectibix) is a human IgG2 antibody that binds to the EGF receptor. Like Cetuximab, it prevents cell signalling by the receptor by blocking the interaction between the receptor and its ligand. It is used in the treatment of colorectal cancer.[58][59]
Rituximab
Rituximab is a chimeric monoclonal IgG1 antibody specific for CD20, developed from its parent antibody Ibritumomab. As with Ibritumomab, Rituximab targets CD20. For this reason it is effective in treating certain types of malignancies that are formed from cancerous B-cells. These include aggressive and indolent lymphomas such as diffuse large B-cell lymphoma and follicular lymphoma, and leukaemias such as B-cell chronic lymphocytic leukaemia. Although the function of CD20 is relatively unknown it has been suggested that CD20 could be a calcium channel involved in B-cell activation. The antibody's mode of action is primarily through the induction of antibody-dependent cell-mediated cytotoxicity and complement-mediated cytotoxicity. Other mechanisms include apoptosis and cellular growth arrest. Rituximab also increases the sensitivity of cancerous B-cells to chemotherapy.[60][61][61][62][63][64]
Tositumomab/iodine (131I) tositumomab regimen
Tositumomab was a murine IgG2a anti-CD20 antibody covalently bound to radioactive Iodine 131 known as "Bexxar" that was approved for treatment of Non-Hodgkin lymphoma. It was withdrawn in February 2014 due to lack of sales.[65][66]
Trastuzumab

Trastuzumab is a monoclonal IgG1 humanized antibody specific for the epidermal growth factor receptor 2 protein (HER2). It received FDA-approval in 1998, and is clinically used for breast cancer. HER-2 is a member of the EGFR family of transmembrane tyrosine kinases. Once activated on cancer cells, it activates cell signalling pathways that promote cell proliferation, cell growth, angiogenesis and metastasis, and inhibits apoptosis.[67]
Amplification or overexpression of HER2 is present in 15-25% of breast carcinomas and is associated with aggressive tumour phenotype, poor prognosis, non-responsiveness to hormonal therapy and reduced sensitivity to conventional chemotherapeutic agents.[67][68] The use of Trastuzumab is restricted to patients whose tumours over-express HER2, primarily assessed by immunohistochemistry (IHC) and Fluorescent in situ hybridisation (FISH). Other methods are less routinely used including PCR-based methodologies and chromogenic in situ hybridization.[69][70]
Cytokine therapy
Cytokines are a broad group of proteins produced by many types of cells present within a tumor. They have the ability to modulate immune responses. The tumor often employs it to allow it to grow and manipulate the immune response. These immune-modulating effects allow them to be used as drugs to provoke an immune response. Two commonly used groups of cytokines are interferons and interleukins.[71]
Interferon
Interferons are cytokines produced by the immune system. They are usually involved in anti-viral response, but also have use for cancer. The three groups of interferons (IFNs) are type I (IFNα and IFNβ), type 2 (IFNγ) and type III (IFNλ). IFNα has been approved for use in hairy-cell leukaemia, AIDS-related Kaposi's sarcoma, follicular lymphoma, chronic myeloid leukaemia and melanoma. Type I and II IFNs have been researched extensively and although both types promote anti-tumor immune system effects, only type I IFNs have been shown to be clinically effective. IFNλ shows promise for its anti-tumor effects in animal models.[72][73]
Interleukin
Interleukins are a group of cytokines with a wide array of immune system effects. Interleukin-2 is used in the treatment of malignant melanoma and renal cell carcinoma. In normal physiology it promotes both effector T cells and T-regulatory cells, but its exact mechanism in the treatment of cancer is unknown.[71][74]
Adjuvant treatment with Polysaccharide-K
Japan's Ministry of Health, Labour and Welfare approved the use of Polysaccharide-K extracted from the mushroom, Coriolus versicolor, in the 1980s, to stimulate the immune systems of patients undergoing chemotherapy. It is a dietary supplement in the US and other jurisdictions.[75]
Research
Adoptive T-cell therapy
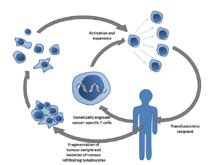
Adoptive T-cell therapy is a form of passive immunization by the transfusion of T-cells. They are found in blood and tissue and usually activate when they find foreign pathogens. Specifically they activate when the T-cell's surface receptors encounter other cells that display small parts of foreign proteins on their surface antigens. These can be either infected cells, or specialised immune cells known as antigen presenting cells (APCs). They are found in normal tissue and in tumor tissue, where they are known as tumor infiltrating lymphocytes (TILs). They are activated by the presence of APCs such as dendritic cells that present tumor antigens. Although these cells have the capability of attacking the tumor, the environment within the tumor is highly immunosuppressive, preventing immune-mediated tumour death.[76]
Multiple ways of producing and obtaining tumour targeted T-cells have been discovered. T-cells specific to a tumor antigen can be removed from a tumor sample (TILs) or filtered from blood. Subsequent activation and culturing is performed outside the body (ex vivo) and then they are transfused into the patient. Activation can take place through gene therapy, or by exposing the T cells to tumor antigens. Although research has made major advances in this form of therapy, no adoptive T-cell therapy is as yet approved.[76][77]
As of 2014, several clinical trials for ACT were underway. Initial clinical trials showed complete remission of leukemia in some patients in two small clinical trials, announced in December 2013.[78][79][80][81][82]
Another approach is adoptive transfer of haploidentical γδ T cells or NK cells from a healthy donor. Major advantage of this approach is that these cells do not cause GVHD. The disadvantage is frequently impaired function of the transferred cells.[83]
Anti-CD47 antibodies
Anti-CD47 antibodies, which block the protein CD47 from telling the cancer host's immune system not to attack it, have been shown to eliminate or inhibit the growth of a wide range of cancers and tumors in laboratory tests on cells and mice. CD47 is present on many cancer cells and on many healthy cells. After the cancer cells have been engulfed by macrophages, the host immune system's CD8+ T Cells become mobilized against the cancer and attack it on their own in addition to the macrophages.[84]
Anti-GD2 antibodies

Carbohydrate antigens on the surface of cells can be used as targets for immunotherapy. GD2 is a ganglioside found on the surface of many types of cancer cell including neuroblastoma, retinoblastoma, melanoma, small cell lung cancer, brain tumors, osteosarcoma, rhabdomyosarcoma, Ewing’s sarcoma, liposarcoma, fibrosarcoma, leiomyosarcoma and other soft tissue sarcomas. It is not usually expressed on the surface of normal tissues, making it a good target for immunotherapy. As of 2014, Phase I, II, and III trials were underway for antibody treatments.[85]
Immune checkpoint blockade
A ligand-receptor interaction that was investigated as a target for cancer treatment is the interaction between the transmembrane programmed cell death 1 protein (PDCD1, PD-1; also known as CD279) and its ligand, PD-1 ligand 1 (PD-L1, CD274). In normal physiology PD-L1 on the cell surface binds to PD1 on an immune cell surface, which inhibits immune cell activity. It appears that upregulation of PD-L1 on the cancer cell surface may allow them to evade the host immune system by inhibiting T cells that might otherwise attack the tumor cell. Antibodies that bind to either PD-1 or PD-L1 and therefore block the interaction may allow the T-cells to attack the tumor. Initial clinical trial results with an IgG4 PD1 antibody called Nivolumab were published in 2010.[86]
EGF receptor antibodies
Development of an anti-EGFR antibody, Matuzumab, was discontinued in 2008.[87][88]
Polysaccharides

Certain compounds found in mushrooms, primarily polysaccharide compounds, can up-regulate the immune system and may have anti-cancer properties. For example, Beta-glucans, such as lentinan have been shown in laboratory studies to stimulate macrophage, NK cells, T cells, and immune system cytokines, and have been investigated in clinical trials as immunologic adjuvant therapies.[89]
Agaricus subrufescens, (often mistakenly called Agaricus blazei), Lentinula edodes (Shiitake mushrooom), Grifola frondosa and Hericium erinaceus are fungi known to produce beta-glucans and have been tested for their anti-cancer potential.[90]
Public awareness
Starting with the FDA approval in 2010 of the therapeutic vaccine sipuleucel-T (Provenge) for prostate cancer and, in 2011, of ipilimumab (Yervoy) for melanoma,[91] public awareness of cancer immunotherapy has increased thanks to mainstream news articles.[92][93][94]
See also
- Antigen 5T4
- Coley's Toxins
External links
- Cancer Research Institute - What is Cancer Immunotherapy
- Association for Immunotherapy of Cancer
- Society for Immunotherapy of Cancer
References
- ↑ Strebhardt, K; Ullrich, A (June 2008). "Paul Ehrlich's magic bullet concept: 100 years of progress.". Nature reviews. Cancer 8 (6): 473–80. doi:10.1038/nrc2394. PMID 18469827.
- ↑ Waldmann, TA (March 2003). "Immunotherapy: past, present and future.". Nature Medicine 9 (3): 269–77. doi:10.1038/nm0303-269. PMID 12612576.
- ↑ 3.0 3.1 Palucka, K; Banchereau, J (Jul 25, 2013). "Dendritic-cell-based therapeutic cancer vaccines.". Immunity 39 (1): 38–48. doi:10.1016/j.immuni.2013.07.004. PMID 23890062.
- ↑ Gardner, TA; Elzey, BD; Hahn, NM (April 2012). "Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer.". Human vaccines & immunotherapeutics 8 (4): 534–9. doi:10.4161/hv.19795. PMID 22832254.
- ↑ Oudard, S (May 2013). "Progress in emerging therapies for advanced prostate cancer.". Cancer treatment reviews 39 (3): 275–89. doi:10.1016/j.ctrv.2012.09.005. PMID 23107383.
- ↑ Sims, RB (Jun 19, 2012). "Development of sipuleucel-T: autologous cellular immunotherapy for the treatment of metastatic castrate resistant prostate cancer.". Vaccine 30 (29): 4394–7. doi:10.1016/j.vaccine.2011.11.058. PMID 22122856.
- ↑ Shore, ND; Mantz, CA; Dosoretz, DE; Fernandez, E; Myslicki, FA; McCoy, C; Finkelstein, SE; Fishman, MN (January 2013). "Building on sipuleucel-T for immunologic treatment of castration-resistant prostate cancer.". Cancer control : journal of the Moffitt Cancer Center 20 (1): 7–16. PMID 23302902.
- ↑ 8.0 8.1 8.2 8.3 Scott, AM; Wolchok, JD; Old, LJ (Mar 22, 2012). "Antibody therapy of cancer.". Nature reviews. Cancer 12 (4): 278–87. doi:10.1038/nrc3236. PMID 22437872.
- ↑ Harding, FA; Stickler, MM; Razo, J; DuBridge, RB (May–Jun 2010). "The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions". MAbs 2 (3): 256–65. doi:10.4161/mabs.2.3.11641. PMID 20400861.
- ↑ Weiner, LM; Surana, R; Wang, S (May 2010). "Monoclonal antibodies: versatile platforms for cancer immunotherapy.". Nature reviews. Immunology 10 (5): 317–27. doi:10.1038/nri2744. PMID 20414205.
- ↑ Seidel, UJ; Schlegel, P; Lang, P (2013). "Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies.". Frontiers in immunology 4: 76. doi:10.3389/fimmu.2013.00076. PMID 23543707.
- ↑ Gelderman, KA; Tomlinson, S; Ross, GD; Gorter, A (March 2004). "Complement function in mAb-mediated cancer immunotherapy.". Trends in immunology 25 (3): 158–64. doi:10.1016/j.it.2004.01.008. PMID 15036044.
- ↑ Sharkey, RM; Goldenberg, DM (March 2011). "Cancer radioimmunotherapy.". Immunotherapy 3 (3): 349–70. doi:10.2217/imt.10.114. PMID 21395378.
- ↑ Waldmann, Thomas A. (2003). "Immunotherapy: past, present and future". Nature Medicine 9 (3): 269–277. doi:10.1038/nm0303-269. PMID 12612576.
- ↑ Demko, S; Summers, J; Keegan, P; Pazdur, R (February 2008). "FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia.". The oncologist 13 (2): 167–74. doi:10.1634/theoncologist.2007-0218. PMID 18305062.
- ↑ Cohen, MH; Gootenberg, J; Keegan, P; Pazdur, R (March 2007). "FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer.". The oncologist 12 (3): 356–61. doi:10.1634/theoncologist.12-3-356. PMID 17405901.
- ↑ Cohen, MH; Gootenberg, J; Keegan, P; Pazdur, R (June 2007). "FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer.". The oncologist 12 (6): 713–8. doi:10.1634/theoncologist.12-6-713. PMID 17602060.
- ↑ Summers, J; Cohen, MH; Keegan, P; Pazdur, R (2010). "FDA drug approval summary: bevacizumab plus interferon for advanced renal cell carcinoma.". The oncologist 15 (1): 104–11. doi:10.1634/theoncologist.2009-0250. PMID 20061402.
- ↑ Cohen, MH; Shen, YL; Keegan, P; Pazdur, R (November 2009). "FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme.". The oncologist 14 (11): 1131–8. doi:10.1634/theoncologist.2009-0121. PMID 19897538.
- ↑ 20.0 20.1 de Claro, RA; McGinn, K; Kwitkowski, V; Bullock, J; Khandelwal, A; Habtemariam, B; Ouyang, Y; Saber, H; Lee, K; Koti, K; Rothmann, M; Shapiro, M; Borrego, F; Clouse, K; Chen, XH; Brown, J; Akinsanya, L; Kane, R; Kaminskas, E; Farrell, A; Pazdur, R (Nov 1, 2012). "U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma.". Clinical cancer research : an official journal of the American Association for Cancer Research 18 (21): 5845–9. doi:10.1158/1078-0432.CCR-12-1803. PMID 22962441.
- ↑ 21.0 21.1 21.2 Pazdur, Richard. "FDA approval for Cetuximab". Retrieved 7 November 2013.
- ↑ Cohen, MH; Chen, H; Shord, S; Fuchs, C; He, K; Zhao, H; Sickafuse, S; Keegan, P; Pazdur, R (2013). "Approval summary: Cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil for the first-line treatment of patients with recurrent locoregional or metastatic squamous cell head and neck cancer.". The oncologist 18 (4): 460–6. doi:10.1634/theoncologist.2012-0458. PMID 23576486.
- ↑ Bross, PF; Beitz, J; Chen, G; Chen, XH; Duffy, E; Kieffer, L; Roy, S; Sridhara, R; Rahman, A; Williams, G; Pazdur, R (June 2001). "Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia.". Clinical cancer research : an official journal of the American Association for Cancer Research 7 (6): 1490–6. PMID 11410481.
- ↑ "FDA - Ibritumomab Tiuxetan". Retrieved 7 November 2013.
- ↑ Pazdur, Richard. "FDA approval for Ipilimumab". Retrieved 7 November 2013.
- ↑ Lemery, SJ; Zhang, J; Rothmann, MD; Yang, J; Earp, J; Zhao, H; McDougal, A; Pilaro, A; Chiang, R; Gootenberg, JE; Keegan, P; Pazdur, R (Sep 1, 2010). "U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab.". Clinical cancer research : an official journal of the American Association for Cancer Research 16 (17): 4331–8. doi:10.1158/1078-0432.CCR-10-0570. PMID 20601446.
- ↑ Giusti, RM; Cohen, MH; Keegan, P; Pazdur, R (March 2009). "FDA review of a panitumumab (Vectibix) clinical trial for first-line treatment of metastatic colorectal cancer.". The oncologist 14 (3): 284–90. doi:10.1634/theoncologist.2008-0254. PMID 19282350.
- ↑ James, JS; Dubs, G (Dec 5, 1997). "FDA approves new kind of lymphoma treatment. Food and Drug Administration.". AIDS treatment news (284): 2–3. PMID 11364912.
- ↑ Casak, SJ; Lemery, SJ; Shen, YL; Rothmann, MD; Khandelwal, A; Zhao, H; Davis, G; Jarral, V; Keegan, P; Pazdur, R (2011). "U.S. Food and drug administration approval: rituximab in combination with fludarabine and cyclophosphamide for the treatment of patients with chronic lymphocytic leukemia.". The oncologist 16 (1): 97–104. doi:10.1634/theoncologist.2010-0306. PMID 21212432.
- ↑ Pazdur, Richard. "FDA Approval for Tositumomab and Iodine I 131 Tositumomab". Retrieved 7 November 2013.
- ↑ "FDA Expands Use of Herceptin for Early Stage Breast Cancer After Primary Therapy". Retrieved 7 November 2013.
- ↑ Byrd JC, Stilgenbauer S, Flinn IW. Chronic Lymphocytic Leukemia. Hematology (Am Soc Hematol Educ Program) 2004: 163-183. Date retrieved: 26/01/2006.
- ↑ Domagała, A; Kurpisz, M (Mar–Apr 2001). "CD52 antigen--a review.". Medical science monitor : international medical journal of experimental and clinical research 7 (2): 325–31. PMID 11257744.
- ↑ Dearden, C (Jul 19, 2012). "How I treat prolymphocytic leukemia.". Blood 120 (3): 538–51. doi:10.1182/blood-2012-01-380139. PMID 22649104.
- ↑ 35.0 35.1 35.2 Kirkwood, JM; Butterfield, LH; Tarhini, AA; Zarour, H; Kalinski, P; Ferrone, S (Sep–Oct 2012). "Immunotherapy of cancer in 2012.". CA: a cancer journal for clinicians 62 (5): 309–35. doi:10.3322/caac.20132. PMID 22576456.
- ↑ Lenz, HJ (April 2005). "Antiangiogenic agents in cancer therapy.". Oncology (Williston Park, N.Y.) 19 (4 Suppl 3): 17–25. PMID 15934499.
- ↑ 37.0 37.1 Gerber, HP; Ferrara, N (Feb 1, 2005). "Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies.". Cancer Research 65 (3): 671–80. PMID 15705858.
- ↑ 38.0 38.1 38.2 Sun, W (Oct 11, 2012). "Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy.". Journal of hematology & oncology 5: 63. doi:10.1186/1756-8722-5-63. PMID 23057939.
- ↑ 39.0 39.1 Mukherji, SK (February 2010). "Bevacizumab (Avastin).". AJNR. American journal of neuroradiology 31 (2): 235–6. doi:10.3174/ajnr.A1987. PMID 20037132.
- ↑ 40.0 40.1 40.2 Cheng, YD; Yang, H; Chen, GQ; Zhang, ZC (Nov 1, 2013). "Molecularly targeted drugs for metastatic colorectal cancer.". Drug design, development and therapy 7: 1315–1322. doi:10.2147/DDDT.S52485. PMID 24204124.
- ↑ Younes, A; Yasothan, U; Kirkpatrick, P (Jan 3, 2012). "Brentuximab vedotin.". Nature reviews. Drug discovery 11 (1): 19–20. doi:10.1038/nrd3629. PMID 22212672.
- ↑ Garnock-Jones, KP (Mar 2013). "Brentuximab vedotin: a review of its use in patients with hodgkin lymphoma and systemic anaplastic large cell lymphoma following previous treatment failure.". Drugs 73 (4): 371–81. doi:10.1007/s40265-013-0031-5. PMID 23494187.
- ↑ Bou-Assaly, W; Mukherji, S (April 2010). "Cetuximab (erbitux).". AJNR. American journal of neuroradiology 31 (4): 626–7. doi:10.3174/ajnr.A2054. PMID 20167650.
- ↑ 44.0 44.1 Ricart, AD (Oct 15, 2011). "Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin.". Clinical cancer research : an official journal of the American Association for Cancer Research 17 (20): 6417–27. doi:10.1158/1078-0432.CCR-11-0486. PMID 22003069.
- ↑ Food and Drug Administration. "Mylotarg (gemtuzumab ozogamicin): Market Withdrawal". Retrieved 23 November 2013.
- ↑ Ravandi, F; Estey, EH; Appelbaum, FR; Lo-Coco, F; Schiffer, CA; Larson, RA; Burnett, AK; Kantarjian, HM (Nov 10, 2012). "Gemtuzumab ozogamicin: time to resurrect?". Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30 (32): 3921–3. doi:10.1200/JCO.2012.43.0132. PMID 22987091.
- ↑ Tennvall, J; Fischer, M; Bischof Delaloye, A; Bombardieri, E; Bodei, L; Giammarile, F; Lassmann, M; Oyen, W; Brans, B; Therapy Committee,, EANM; Oncology Committee,, EANM; Dosimetry Committee,, EANM (April 2007). "EANM procedure guideline for radio-immunotherapy for B-cell lymphoma with 90Y-radiolabelled ibritumomab tiuxetan (Zevalin).". European journal of nuclear medicine and molecular imaging 34 (4): 616–22. doi:10.1007/s00259-007-0372-y. PMID 17323056.
- ↑ Maloney, DG (May 24, 2012). "Anti-CD20 antibody therapy for B-cell lymphomas.". The New England Journal of Medicine 366 (21): 2008–16. doi:10.1056/NEJMct1114348. PMID 22621628.
- ↑ 49.0 49.1 Sondak, VK; Smalley, KS; Kudchadkar, R; Grippon, S; Kirkpatrick, P (Jun 2011). "Ipilimumab.". Nature reviews. Drug discovery 10 (6): 411–2. doi:10.1038/nrd3463. PMID 21629286.
- ↑ 50.0 50.1 Lipson, EJ; Drake, CG (Nov 15, 2011). "Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma.". Clinical cancer research : an official journal of the American Association for Cancer Research 17 (22): 6958–62. doi:10.1158/1078-0432.CCR-11-1595. PMID 21900389.
- ↑ 51.0 51.1 Thumar, JR; Kluger, HM (Dec 2010). "Ipilimumab: a promising immunotherapy for melanoma.". Oncology (Williston Park, N.Y.) 24 (14): 1280–8. PMID 21294471.
- ↑ 52.0 52.1 Chambers, CA; Kuhns, MS; Egen, JG; Allison, JP (2001). "CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy.". Annual review of immunology 19: 565–94. doi:10.1146/annurev.immunol.19.1.565. PMID 11244047.
- ↑ John Dorschner for phys.org. September 04, 2009 Cuban cancer drug undergoes rare U.S. trial
- ↑ Ramakrishnan, Melarkode S.; Anand Eswaraiah, Tania Crombet, Patricia Piedra, Giselle Saurez, Harish Iyer and A.S. Arvind (2009). "Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin". mAbs 1 (1): 41–48. doi:10.4161/mabs.1.1.7509. PMC 2715181. PMID 20046573.
- ↑ Vacchelli E et al. (Jan 2014). "Trial Watch: Tumor-targeting monoclonal antibodies in cancer therapy". Oncoimmunology 3 (1): e27048. doi:10.4161/onci.27048. PMID 24605265.
- ↑ Castillo, J; Perez, K (2010). "The role of ofatumumab in the treatment of chronic lymphocytic leukemia resistant to previous therapies.". Journal of blood medicine 1: 1–8. doi:10.2147/jbm.s7284. PMC 3262337. PMID 22282677.
- ↑ Zhang, B (Jul–Aug 2009). "Ofatumumab.". mAbs 1 (4): 326–31. doi:10.4161/mabs.1.4.8895. PMC 2726602. PMID 20068404.
- ↑ Keating, GM (May 28, 2010). "Panitumumab: a review of its use in metastatic colorectal cancer.". Drugs 70 (8): 1059–78. doi:10.2165/11205090-000000000-00000. PMID 20481659.
- ↑ Saltz, L; Easley, C; Kirkpatrick, P (Dec 2006). "Panitumumab.". Nature reviews. Drug discovery 5 (12): 987–8. doi:10.1038/nrd2204. PMID 17201026.
- ↑ Keating, GM (Jul 30, 2010). "Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma.". Drugs 70 (11): 1445–76. doi:10.2165/11201110-000000000-00000. PMID 20614951.
- ↑ 61.0 61.1 Plosker, GL; Figgitt, DP (2003). "Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.". Drugs 63 (8): 803–43. doi:10.2165/00003495-200363080-00005. PMID 12662126.
- ↑ Cerny, T; Borisch, B; Introna, M; Johnson, P; Rose, AL (Nov 2002). "Mechanism of action of rituximab.". Anti-cancer drugs. 13 Suppl 2: S3–10. doi:10.1097/00001813-200211002-00002. PMID 12710585.
- ↑ Janeway, Charles; Paul Travers; Mark Walport; Mark Shlomchik (2001). Immunobiology; Fifth Edition. New York and London: Garland Science. ISBN 0-8153-4101-6.
- ↑ Weiner, GJ (Apr 2010). "Rituximab: mechanism of action.". Seminars in hematology 47 (2): 115–23. doi:10.1053/j.seminhematol.2010.01.011. PMC 2848172. PMID 20350658.
- ↑ "Why Good Drugs Sometimes Fail: The Bexxar Story".
- ↑ Notice by the Food and Drug Administration on October 23, 2013. GlaxoSmithKline LLC; Withdrawal of Approval of the Indication for Treatment of Patients With Relapsed or Refractory, Low Grade, Follicular, or Transformed CD20 Positive Non-Hodgkin's Lymphoma Who Have Not Received Prior Rituximab; BEXXAR
- ↑ 67.0 67.1 Garnock-Jones, KP; Keating, GM; Scott, LJ (2010). "Trastuzumab: A review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer.". Drugs 70 (2): 215–39. doi:10.2165/11203700-000000000-00000. PMID 20108993.
- ↑ Slamon, DJ; Godolphin, W; Jones, LA; Holt, JA; Wong, SG; Keith, DE; Levin, WJ; Stuart, SG et al. (1989). "Studies of the HER-2/neu proto oncogene in human breast and ovarian cancer". Science 244 (4905): 707–712. doi:10.1126/science.2470152. PMID 2470152.
- ↑ Shaaban, AM; Purdie, CA; Bartlett, JM; Stein, RC; Lane, S; Francis, A; Thompson, AM; Pinder, SE; Translational Subgroup of the NCRI Breast Clinical Studies, Group (Feb 2014). "HER2 testing for breast carcinoma: recommendations for rapid diagnostic pathways in clinical practice.". Journal of clinical pathology 67 (2): 161–7. doi:10.1136/jclinpath-2013-201819. PMID 24062360.
- ↑ van de Vijver, M (2002). "Emerging technologies for HER2 testing.". Oncology. 63 Suppl 1: 33–8. doi:10.1159/000066199. PMID 12422053.
- ↑ 71.0 71.1 Dranoff, G (Jan 2004). "Cytokines in cancer pathogenesis and cancer therapy.". Nature reviews. Cancer 4 (1): 11–22. doi:10.1038/nrc1252. PMID 14708024.
- ↑ Dunn, GP; Koebel, CM; Schreiber, RD (November 2006). "Interferons, immunity and cancer immunoediting.". Nature reviews. Immunology 6 (11): 836–48. doi:10.1038/nri1961. PMID 17063185.
- ↑ Lasfar, A; Abushahba, W; Balan, M; Cohen-Solal, KA (2011). "Interferon lambda: a new sword in cancer immunotherapy.". Clinical & developmental immunology 2011: 349575. doi:10.1155/2011/349575. PMID 22190970.
- ↑ Coventry, BJ; Ashdown, ML (2012). "The 20th anniversary of interleukin-2 therapy: bimodal role explaining longstanding random induction of complete clinical responses.". Cancer management and research 4: 215–21. doi:10.2147/cmar.s33979. PMC 3421468. PMID 22904643.
- ↑ American Cancer Society. Coriolus Versicolor Last Medical Review: 11/01/2008. Last Revised: 11/01/2008. Accessed May 10, 2014
- ↑ 76.0 76.1 Restifo, NP; Dudley, ME; Rosenberg, SA (Mar 22, 2012). "Adoptive immunotherapy for cancer: harnessing the T cell response.". Nature reviews. Immunology 12 (4): 269–81. doi:10.1038/nri3191. PMID 22437939.
- ↑ June, CH (June 2007). "Adoptive T cell therapy for cancer in the clinic.". The Journal of Clinical Investigation 117 (6): 1466–76. doi:10.1172/JCI32446. PMC 1878537. PMID 17549249.
- ↑ John Carroll for Fierce Biotech. December 9, 2013 Novartis/Penn's customized T cell wows ASH with stellar leukemia data
- ↑ John Carroll for FierceBiotech February 18, 2014 Servier stages an entry into high-stakes CAR-T showdown with Novartis
- ↑ Damian Garde for FierceBiotech. April 24, 2014 Juno wraps up a $176M A round as it hits the gas on its CAR-T contender
- ↑ "CAR T-Cell Therapy: Engineering Patients’ Immune Cells to Treat Their Cancers". cancer.gov. 2013-12-06. Retrieved 2014-05-09.
- ↑ "NIH study demonstrates that a new cancer immunotherapy method could be effective against a wide range of cancers". nih.gov. 2014-05-08. Retrieved 2014-05-09.
- ↑ Wilhelm M (Feb 2014). "Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells.". J Transl Med. 12: 45. doi:10.1186/1479-5876-12-45. PMC 3926263. PMID 24528541.
- ↑ Unanue, ER (Jul 2, 2013). "Perspectives on anti-CD47 antibody treatment for experimental cancer.". Proceedings of the National Academy of Sciences of the United States of America 110 (27): 10886–7. doi:10.1073/pnas.1308463110. PMC 3704033. PMID 23784781.
- ↑ Ahmed, M; Cheung, NK (Jan 21, 2014). "Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy.". FEBS Letters 588 (2): 288–97. doi:10.1016/j.febslet.2013.11.030. PMID 24295643.
- ↑ Pardoll, DM (Mar 22, 2012). "The blockade of immune checkpoints in cancer immunotherapy.". Nature reviews. Cancer 12 (4): 252–64. doi:10.1038/nrc3239. PMID 22437870.
- ↑ "Merck KGaA stellt Entwicklung von Matuzumab ein" [Merck KGaA discontinues development of matuzumab] (in German). NewsVZ.de. February 18, 2008. Retrieved January 5, 2010.
- ↑ "Takeda Discontinues Development of Matuzumab". Takeda Pharmaceutical Co., Ltd. February 18, 2008. Retrieved January 5, 2010.
- ↑ Chen J et al. (Jun 2013). "The application of fungal β-glucans for the treatment of colon cancer". Anticancer Agents Med Chem. 13 (5): 725–30. doi:10.2174/1871520611313050007. PMID 23140353.
- ↑ Patel, S; Goyal, A (Mar 2012). "Recent developments in mushrooms as anti-cancer therapeutics: a review.". 3 Biotech 2 (1): 1–15. doi:10.1007/s13205-011-0036-2. PMC 3339609. PMID 22582152.
- ↑ Lacroix, Marc (2014). Targeted Therapies in Cancer. Hauppauge , NY: Nova Sciences Publishers. ISBN 978-1-63321-687-7.
- ↑ Grady, Denise (2012-12-09). 10, 2012/health/a-breakthrough-against-leukemia-using-altered-t-cells.html?pagewanted=all "A Breakthrough Against Leukemia Using Altered T-Cells". The New York Times.
- ↑ Winslow, Ron (2013-05-15). "New Cancer Drugs Harness Power of Immune System - WSJ.com". Online.wsj.com. Retrieved 2013-08-25.
- ↑ Denise Grady for the New York Times. May 8, 2014. Patient’s Cells Deployed to Attack Aggressive Cancer
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||