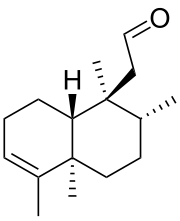
Callicarpenal
 | |
| Names | |
|---|---|
| IUPAC name
[(1S,2R,4aR,8aR)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8,8a-octahydro-1-naphthalenyl]acetaldehyde | |
| Other names
(−)-Callicarpenal; 13,14,15,16-Tetranor-3-cleroden-12-al | |
| Identifiers | |
| 161105-12-0 | |
| ChemSpider | 9282422 |
| |
| Jmol-3D images | Image |
| PubChem | 11107286 |
| |
| Properties | |
| Molecular formula |
C16H26O |
| Molar mass | 234.38 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Callicarpenal is a terpenoid that has been isolated from plants of the genus Callicarpa (beautyberry).[1] It acts as an insect repellent against mosquitoes (Aedes aegypti and Anopheles stephensi) and fire ants.[1][2] It also has activity against ticks (Ixodes scapularis and Amblyomma americanum).[3]
In comparison to the most commonly used insect repellent, DEET, callicarpenal is less effective against mosquitoes.[4]
Callicapenal was discovered by scientists at the United States Department of Agriculture's Agricultural Research Service who were inspired by reports that Callicarpa americana (American beautyberry) was used as a folk remedy to prevent mosquito bites.[5]
References
- ↑ 1.0 1.1 Cantrell, C. L.; Klun, J. A.; Bryson, C. T.; Kobaisy, M.; Duke, S. O. (2005). "Isolation and Identification of Mosquito Bite Deterrent Terpenoids from Leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) Beautyberry". Journal of Agricultural and Food Chemistry 53 (15): 5948–53. doi:10.1021/jf0509308. PMID 16028979.
- ↑ Chen, J; Cantrell, CL; Duke, SO; Allen, ML (2008). "Repellency of callicarpenal and intermedeol against workers of imported fire ants (Hymenoptera: Formicidae)". Journal of economic entomology 101 (2): 265–71. doi:10.1603/0022-0493(2008)101[265:ROCAIA]2.0.CO;2. PMID 18459387.
- ↑ Carroll, JF; Cantrell, CL; Klun, JA; Kramer, M (2007). "Repellency of two terpenoid compounds isolated from Callicarpa americana (Lamiaceae) against Ixodes scapularis and Amblyomma americanum ticks". Experimental & applied acarology 41 (3): 215–24. doi:10.1007/s10493-007-9057-2. PMID 17380408.
- ↑ Ling, Taotao; Xu, Jing; Smith, Ryan; Ali, Abbas; Cantrell, Charles L.; Theodorakis, Emmanuel A. (2011). "Synthesis of (−)-callicarpenal, a potent arthropod repellent". Tetrahedron 67 (17): 3023–3029. doi:10.1016/j.tet.2011.02.078. PMC 3105892. PMID 21643472.
- ↑ Luis Pons (February 2006). "Folk Remedy Yields Mosquito-Thwarting Compound". Agricultural Research Magazine (U.S. Department of Agriculture) 54 (2).
External links
- "Scientists Confirm Folk Remedy Repels Mosquitoes". ScienceDaily. July 3, 2006.