Calcitriol
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
| (1R,3S)-5-[2-[(1R,3aR,7aS)-1-[(2R)-6-hydroxy-6-methyl-heptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H- inden-4-ylidene]ethylidene]-4-methylidene-cyclohexane-1,3-diol | |
| Clinical data | |
| Trade names | Rocaltrol, Calcijex, Decostriol |
| MedlinePlus | a682335 |
| |
| Oral, IV, topical | |
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Renal |
| Half-life | 5–8 hours (adults), 27 hours (children) |
| Excretion | Faeces (50%), urine (16%) |
| Identifiers | |
|
32222-06-3 | |
| A11CC04 D05AX03 | |
| PubChem | CID 5280453 |
| IUPHAR ligand | 2779 |
| DrugBank |
DB00136 |
| ChemSpider |
4444108 |
| UNII |
FXC9231JVH |
| ChEBI |
CHEBI:17823 |
| ChEMBL |
CHEMBL846 |
| Chemical data | |
| Formula | C27H44O3 |
| 416.64 g/mol | |
|
SMILES
| |
| |
| | |
Calcitriol (INN) /kælˈsɪtri.ɒl/, also called 1,25-dihydroxycholecalciferol or 1,25-dihydroxyvitamin D3, is the hormonally active metabolite of vitamin D with three hydroxyl groups (abbreviated 1,25-(OH)2D3 or simply 1,25(OH)2D),[1] It was first identified by Michael F. Holick in work published in 1971.[2] Calcitriol increases the level of calcium (Ca2+) in the blood by increasing the uptake of calcium from the gut into the blood, and possibly increasing the release of calcium into the blood from bone.[3]
Nomenclature
Calcitriol usually refers specifically to 1,25-dihydroxycholecalciferol, but may also sometimes include 24,25-dihydroxycholecalciferol (when specified), the deactivated form of vitamin D.
Because cholecalciferol already has one hydroxyl group, only two are further specified in the nomenclature.
Pharmaceutical trade names
Calcitriol is marketed under various trade names including Rocaltrol (Roche), Calcijex (Abbott), Decostriol (Mibe, Jesalis), Biowoz (Solmarc) and Vectical (Galderma), Rolsical (Sun Pharma).
Function
Calcitriol increases blood calcium levels ([Ca2+]) by promoting absorption of dietary calcium from the gastrointestinal tract and increasing renal tubular reabsorption of calcium, thus reducing the loss of calcium in the urine. Calcitriol also stimulates release of calcium from bone by its action on the specific type of bone cells referred to as osteoblasts, causing them to release RANKL, which in turn activates osteoclasts.[4]
Calcitriol acts in concert with parathyroid hormone (PTH) in all three of these roles. For instance, PTH also indirectly stimulates osteoclasts. However, the main effect of PTH is to increase the rate at which the kidneys excrete inorganic phosphate (Pi), the counterion of Ca2+. The resulting decrease in serum phosphate causes hydroxyapatite (Ca5(PO4)3OH) to dissolve out of bone thus increasing serum calcium. PTH also stimulates the production of calcitriol (see below).[3]
Many of the effects of calcitriol are mediated by its interaction with the calcitriol receptor, also called the vitamin D receptor or VDR. For instance, the unbound inactive form of the calcitriol receptor in intestinal epithelial cells resides in the cytoplasm. When calcitriol binds to the receptor, the ligand-receptor complex translocates to the cell nucleus, where it acts as a transcription factor promoting the expression of a gene encoding a calcium binding protein. The levels of the calcium binding protein increase enabling the cells to actively transport more calcium (Ca2+) from the intestine across the intestinal mucosa into the blood.[3]
The maintenance of electroneutrality requires that the transport of Ca2+ ions catalyzed by the intestinal epithelial cells be accompanied by counterions, primarily inorganic phosphate. Thus calcitriol also stimulates the intestinal absorption of phosphate.[3]
The observation that calcitriol stimulates the release of calcium from bone seems contradictory, given that sufficient levels of serum calcitriol generally prevent overall loss of calcium from bone. It is believed that the increased levels of serum calcium resulting from calcitriol-stimulated intestinal uptake causes bone to take up more calcium than it loses by hormonal stimulation of osteoclasts.[3] Only when there are conditions, such as dietary calcium deficiency or defects in intestinal transport, which result in a reduction of serum calcium does an overall loss of calcium from bone occur.
Calcitriol also inhibits the release of calcitonin, a hormone which reduces blood calcium primarily by inhibiting calcium release from bone.[3] (The effect of calcitonin on renal excretion is disputed.)[5]
Biosynthesis and its regulation
Calcitriol is produced in the cells of the proximal tubule of the nephron in the kidneys by the action of 25-hydroxyvitamin D3 1-alpha-hydroxylase, a mitochondrial oxygenase and an enzyme which catalyzes the hydroxylation of 25-hydroxycholecalciferol (calcifediol). The activity of the enzyme is stimulated by PTH. The reaction is an important control point in Ca2+ homeostasis.[3]
The production of calcitriol is also increased by prolactin, a hormone which stimulates lactogenesis (the formation of milk in mammary glands), a process which requires large amounts of calcium. It is decreased by high levels of serum phosphate and by an increase in the production of the hormone FGF-23 by osteocyte cells in bone.
Metabolism
Calcitriol becomes calcitroic acid through the action of 24-hydroxylase. Calcitroic acid is excreted in the urine.
Medical use
Calcitriol is prescribed for:[6]
- Treatment of hypocalcaemia – hypoparathyroidism, osteomalacia (adults), rickets (infants, children), renal osteodystrophy, chronic kidney disease
- Treatment of osteoporosis
- Prevention of corticosteroid-induced osteoporosis
Calcitriol is also sometimes used topically in the treatment of psoriasis, however the evidence to support its efficacy is not well established.[7] The vitamin D analogue calcipotriol is more commonly used for psoriasis. Research on the noncalcemic actions of calcitriol and other VDR-ligand analogs and their possible therapeutic applications has been reviewed.[8]
Calcitriol is also administered orally for the treatment of psoriasis[9] and psoriatic arthritis.[10]
Adverse effects
The main adverse drug reaction associated with calcitriol therapy is hypercalcaemia – early symptoms include: nausea, vomiting, constipation, anorexia, apathy, headache, thirst, sweating, and/or polyuria. Compared to other vitamin D compounds in clinical use (cholecalciferol, ergocalciferol), calcitriol has a higher risk of inducing hypercalcaemia. However, such episodes may be shorter and easier to treat due to its relatively short half-life.[6]
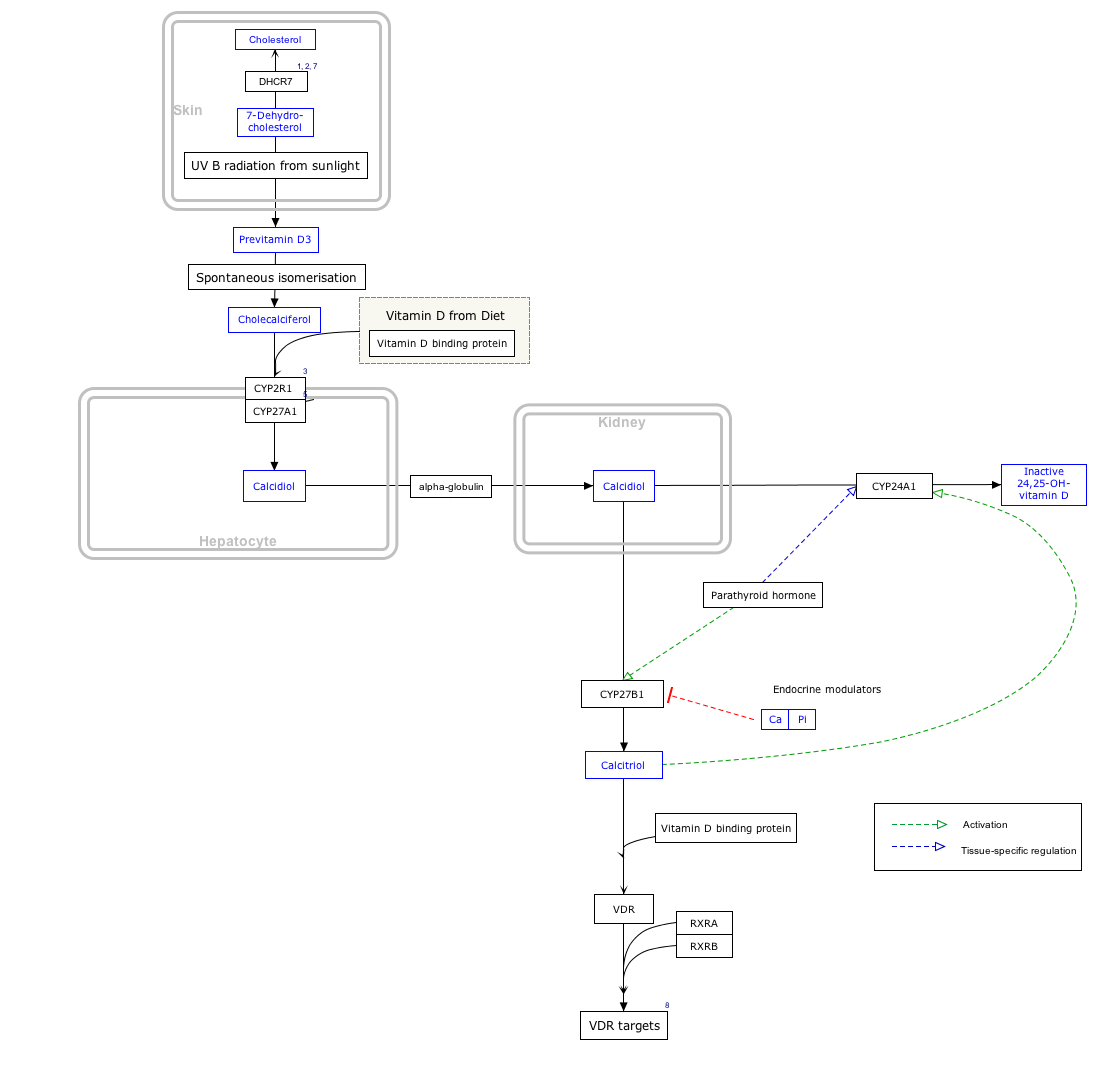
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
Vitamin D Synthesis Pathway edit
- ↑ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
Additional images
-

Calcitriol synthesis
See also
References
- ↑ "Nomenclature of Vitamin D. Recommendations 1981. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)" reproduced at the Queen Mary, University of London website. Retrieved 21 March 2010.
- ↑ Holick, MF; Schnoes, HK; Deluca, HF; Suda, T; Cousins, RJ (1971). "Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine". Biochemistry 10 (14): 2799–804. doi:10.1021/bi00790a023. PMID 4326883.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Voet, Donald; Voet, Judith G. (2004). Biochemistry. Volume one. Biomolecules, mechanisms of enzyme action, and metabolism, 3rd edition, pp. 663–664. New York: John Wiley & Sons. ISBN 0-471-25090-2.
- ↑ Bringhurst, F. R; Demay, Marie B.; Krane, Stephen M.; Kronenberg, Henry M. "Bone and Mineral Metabolism in Health and Disease", chapter 346 in Fauci, Anthony S.; Braunwald, E.; Kasper, D. L.; Hauser, S. L.; Longo D. L.; Jameson, J. L.; Loscalzo, J. (2008). Harrison's Principles of Internal Medicine, 17th edition. New York: McGraw-Hill. ISBN 978-0-07-159991-7. Available online at AccessMedicine.com, purchase required.
- ↑ Carney SL (1997). "Calcitonin and human renal calcium and electrolyte transport". Miner Electrolyte Metab 23 (1): 43–7. PMID 9058369.
- ↑ 6.0 6.1 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ Calcitriol. In: Klasco RK, editor. Drugdex system. vol 128. Greenwood Village (CO): Thomson Micromedex; 2006.
- ↑ Nagpal, S.; Na, S.; Rathnachalam, R. (2005). "Noncalcemic Actions of Vitamin D Receptor Ligands". Endocrine Reviews 26 (5): 662–687. doi:10.1210/er.2004-0002. PMID 15798098..
- ↑ Smith, E. L.; Pincus, S. H.; Donovan, L.; Holick, M. F. (1988). "A novel approach for the evaluation and treatment of psoriasis". Journal of the American Academy of Dermatology 19 (3): 516–528. doi:10.1016/S0190-9622(88)70207-8. PMID 2459166.
- ↑ Huckins, D.; Felson, D. T.; Holick, M. (1990). "Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: A pilot study". Arthritis & Rheumatism 33 (11): 1723. doi:10.1002/art.1780331117.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||