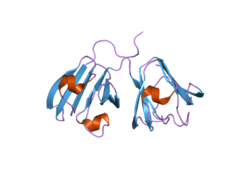CRYGB
Gamma-crystallin B is a protein that in humans is encoded by the CRYGB gene.[1]
Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a superfamily. Alpha and beta families are further divided into acidic and basic groups. Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Gamma-crystallins are a homogeneous group of highly symmetrical, monomeric proteins typically lacking connecting peptides and terminal extensions. They are differentially regulated after early development. Four gamma-crystallin genes (gamma-A through gamma-D) and three pseudogenes (gamma-E, gamma-F, gamma-G) are tandemly organized in a genomic segment as a gene cluster. Whether due to aging or mutations in specific genes, gamma-crystallins have been involved in cataract formation.[1]
References
Further reading
- Graw J (1998). "The crystallins: genes, proteins and diseases". Biol. Chem. 378 (11): 1331–48. PMID 9426193.
- Slingsby C, Clout NJ (2000). "Structure of the crystallins". Eye (London, England). 13 ( Pt 3b): 395–402. PMID 10627816.
- Hearne CM, Todd JA (1991). "Trinucleotide repeat polymorphism at the CRYG1 locus". Nucleic Acids Res. 19 (19): 5450. doi:10.1093/nar/19.19.5450-a. PMC 328932. PMID 1923840.
- Brakenhoff RH; Aarts HJ; Reek FH et al. (1991). "Human gamma-crystallin genes. A gene family on its way to extinction". J. Mol. Biol. 216 (3): 519–32. doi:10.1016/0022-2836(90)90380-5. PMID 2258929.
- den Dunnen JT; van Neck JW; Cremers FP et al. (1989). "Nucleotide sequence of the rat gamma-crystallin gene region and comparison with an orthologous human region". Gene 78 (2): 201–13. doi:10.1016/0378-1119(89)90223-0. PMID 2777080.
- Shiloh Y; Donlon T; Bruns G et al. (1986). "Assignment of the human gamma-crystallin gene cluster (CRYG) to the long arm of chromosome 2, region q33-36". Hum. Genet. 73 (1): 17–9. doi:10.1007/BF00292656. PMID 3011643.
- Meakin SO, Du RP, Tsui LC, Breitman ML (1987). "Gamma-crystallins of the human eye lens: expression analysis of five members of the gene family". Mol. Cell. Biol. 7 (8): 2671–9. PMC 367883. PMID 3670288.
- den Dunnen JT, Moormann RJ, Cremers FP, Schoenmakers JG (1986). "Two human gamma-crystallin genes are linked and riddled with Alu-repeats". Gene 38 (1–3): 197–204. doi:10.1016/0378-1119(85)90218-5. PMID 4065573.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Héon E; Priston M; Schorderet DF et al. (1999). "The gamma-crystallins and human cataracts: a puzzle made clearer". Am. J. Hum. Genet. 65 (5): 1261–7. doi:10.1086/302619. PMC 1288278. PMID 10521291.
- Santhiya ST; Shyam Manohar M; Rawlley D et al. (2002). "Novel mutations in the gamma-crystallin genes cause autosomal dominant congenital cataracts". J. Med. Genet. 39 (5): 352–8. doi:10.1136/jmg.39.5.352. PMC 1735119. PMID 12011157.
- MacCoss MJ; McDonald WH; Saraf A et al. (2002). "Shotgun identification of protein modifications from protein complexes and lens tissue". Proc. Natl. Acad. Sci. U.S.A. 99 (12): 7900–5. doi:10.1073/pnas.122231399. PMC 122992. PMID 12060738.
- Strausberg RL; Feingold EA; Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Salim A, Zaidi ZH (2003). "Homology models of human gamma-crystallins: structural study of the extensive charge network in gamma-crystallins". Biochem. Biophys. Res. Commun. 300 (3): 624–30. doi:10.1016/S0006-291X(02)02895-4. PMID 12507494.
- Nandrot E; Slingsby C; Basak A et al. (2003). "Gamma-D crystallin gene (CRYGD) mutation causes autosomal dominant congenital cerulean cataracts". J. Med. Genet. 40 (4): 262–7. doi:10.1136/jmg.40.4.262. PMC 1735438. PMID 12676897.
- Lapko VN, Smith DL, Smith JB (2004). "Methylation and carbamylation of human gamma-crystallins". Protein Sci. 12 (8): 1762–74. doi:10.1110/ps.0305403. PMC 2323962. PMID 12876325.
- Salim A, Bano A, Zaidi ZH (2004). "Prediction of possible sites for posttranslational modifications in human gamma crystallins: effect of glycation on the structure of human gamma-B-crystallin as analyzed by molecular modeling". Proteins 53 (2): 162–73. doi:10.1002/prot.10493. PMID 14517968.
- Gerhard DS; Wagner L; Feingold EA et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
|
|---|
| | Opsin (retinylidene protein) | |
|---|
| | Crystallin | |
|---|
| | Other | |
|---|
| |
|---|
| | Description |
- Anatomy
- Physiology
- Phenomena
- appearance
- visual
- optical illusions
- proteins
- Development
|
|---|
| | Disease |
- Congenital
- Corneal dystrophy
- Neoplasms and cancer
- Other
- Symptoms and signs
|
|---|
| | Treatment |
- Procedures
- Drugs
- infection
- glaucoma and miosis
- mydriatics
- vascular
|
|---|
|
|