COPII
COPII is a type of vesicle coat protein that transports proteins from the rough endoplasmic reticulum to the Golgi apparatus.[2][3] This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI protein. The name "COPII" refers to the specific coat protein complex that initiates the budding process. The coat consists of large protein subcomplexes that are made of four different protein subunits.
Coat proteins
There are two protein heterodimers that form the coat complex. These proteins are
- Sec23p/Sec24p Heterodimer
- Sec13p/Sec31p Heterotetramer
These proteins alone are not able to cause the budding of the vesicle or direct the vesicle to the correct target membrane. SNARE, cargo, and other proteins are also needed for these processes to occur. The CopII protein does, however, cause the binding that forms vesicle coat, and thereby causes the release from the ER. The exact process of how the vesicle is brought to a particular location, or how that location is determined is not yet known.
Budding process
The GTPase Sar1p is a protein that hydrolyzes GTP and acts like a molecular "switch" that flips between an activated and membrane embedded GTP-bound form, and inactive and soluble GDP-bound form. Inactive GDP-bound Sar1p is attracted to the cytosolic side of the endoplasmic reticulum. Sec12, a transmembrane protein found in the ER acts as a Guanine nucleotide exchange factor by stimulating the release of GDP to allow the binding of GTP. Now in a GTP bound state, Sar1p undergoes a conformational change which exposes a hydrophobic tail that can be inserted into the lipid bilayer, binding it to the membrane. Once Sar1p is bound to the membrane the coat protein complexes Sec23p/24p and Sec13p/31p bind to the membrane sequentially. These proteins simultaneously contact Sar1p and cargo proteins destined for the cis-golgi membrane. The Sec23p/24p-Sec13p/31p-Sar1p complexes then coalesce to form a much larger complex. This network deforms the membrane enough to bud a vesicle off.
Conformational changes
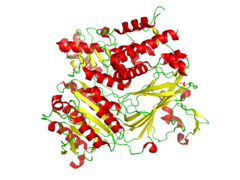
CopII has three specific binding sites that can each be complexed. The picture on the right (Sed5) uses the Sec22 t-SNARE complex to bind. This site is more strongly bound, and therefore is more favored. (Embo)
| Crystal structures of CopII |
|---|
| Conformation of the CopII protein that is complexed with the snare protein Sed5 ( PDB 1PD0). [4] |
|
See also
References
|
|---|
| | Synaptic vesicle | |
|---|
| | COPI | |
|---|
| | COPII | |
|---|
| | RME/Clathrin | |
|---|
| | Caveolae | |
|---|
| | Other/ungrouped | | Vesicle formation | | Adaptor protein complex 1: | |
|---|
| | Adaptor protein complex 2: | |
|---|
| | Adaptor protein complex 3: | |
|---|
| | Adaptor protein complex 4: | |
|---|
| | | | | | BLOC-1: | |
|---|
| | BLOC-2: | |
|---|
| | BLOC-3: | |
|---|
| | Coats: | |
|---|
|
|---|
| | Small GTPase | |
|---|
| | Other | |
|---|
|
|---|
| See also vesicular transport protein disorders
Index of cells |
|---|
| | Description |
- Structure
- Organelles
- peroxisome
- cytoskeleton
- centrosome
- epithelia
- cilia
- mitochondria
- Membranes
- Membrane transport
- ion channels
- vesicular transport
- solute carrier
- ABC transporters
- ATPase
- oxidoreduction-driven
|
|---|
| | Disease |
- Structural
- peroxisome
- cytoskeleton
- cilia
- mitochondria
- nucleus
- scleroprotein
- Membrane
- channelopathy
- solute carrier
- ATPase
- ABC transporters
- other
- extracellular ligands
- cell surface receptors
- intracellular signalling
- Vesicular transport
- Pore-forming toxins
|
|---|
|
|