CERC-301
 | |
| Names | |
|---|---|
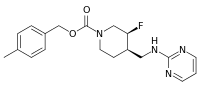
| IUPAC name
4-Methylbenzyl (3S,4R)-3-fluoro-4-[(2-pyrimidinylamino)methyl]-1-piperidinecarboxylate | |
| Identifiers | |
| ChemSpider | 9569140 |
| |
| Jmol-3D images | Image |
| PubChem | 11394238 |
| |
| Properties | |
| Molecular formula |
C19H23FN4O2 |
| Molar mass | 358.41 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
CERC-301 (formerly known as MK-0657) is an orally-active, selective NMDA receptor subunit 2B (NR2B) antagonist which is under development by Cerecor in the United States as an adjunctive therapy for treatment-resistant depression (TRD).[1] In November 2013, phase II clinical trials were initiated,[2] and in the same month, CERC-301 received Fast Track Designation from the Food and Drug Administration for TRD.[3]
A pilot study was published in 2012,[1] and a phase 2 trial was completed in 2014.[4]
See also
- Esketamine
- Rapastinel
- NRX-1074
- ALKS-5461
- LY-2456302
- NSI-189
References
- ↑ 1.0 1.1 Ibrahim L, Diaz Granados N, Jolkovsky L et al. (August 2012). "A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder". J Clin Psychopharmacol 32 (4): 551–7. doi:10.1097/JCP.0b013e31825d70d6. PMC 3438886. PMID 22722512.
- ↑ Yahoo! Finance (2013). "Cerecor Announces Initiation of Phase 2 Study for CERC-301, an Oral Rapid-acting Antidepressant Candidate".
- ↑ Yahoo! Finance (2013). "Cerecor Receives Fast Track Designation for CERC-301 for the Treatment of Major Depressive Disorder".
- ↑ "A Randomized, Double-Blind, Placebo-Controlled, Sequential Parallel Study of CERC-301 in the Adjunctive Treatment of Subjects With Severe Depression and Recent Active Suicidal Ideation Despite Antidepressant Treatment". Retrieved 2015-01-04.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||