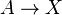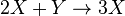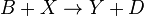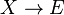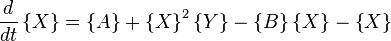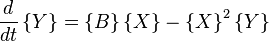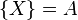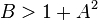Brusselator
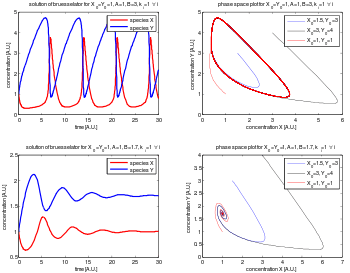

The Brusselator is a theoretical model for a type of autocatalytic reaction. The Brusselator model was proposed by Ilya Prigogine and his collaborators at the Université Libre de Bruxelles.[1] It is a portmanteau of Brussels and oscillator.
It is characterized by the reactions
Under conditions where A and B are in vast excess and can thus can be modeled at constant concentration, the rate equations become
where, for convenience, the rate constants have been set to 1.
The Brusselator has a fixed point at
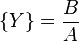 .
.
The fixed point becomes unstable when
leading to an oscillation of the system. Unlike the Lotka–Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.[2]
The best-known example is the clock reaction, the Belousov–Zhabotinsky reaction (BZ reaction). It can be created with a mixture of potassium bromate 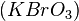 , malonic acid
, malonic acid 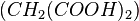 , and manganese sulfate
, and manganese sulfate 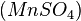 prepared in a heated solution of sulfuric acid
prepared in a heated solution of sulfuric acid 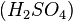 .[3]
.[3]
See also
References
- ↑ "IDEA - Internet Differential Equations Activities". Washington State University. Retrieved 2010-05-16.
- ↑ http://www.bibliotecapleyades.net/archivos_pdf/brusselator.pdf Dynamics of the Brusselator
- ↑ BZ reaction