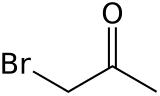
Bromoacetone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1-Bromoacetone | |
| Other names
Bromoacetone 1-Bromo-2-propanone α-Bromoacetone Acetonyl bromide Acetyl methyl bromide Bromomethyl methyl ketone Monobromoacetone Martonite BA UN 1569 | |
| Identifiers | |
| 598-31-2 | |
| ChEBI | CHEBI:51845 |
| ChEMBL | ChEMBL1085947 |
| ChemSpider | 11223 |
| |
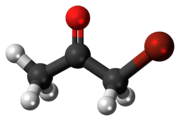
| Jmol-3D images | Image |
| PubChem | 11715 |
| RTECS number | UC0525000 |
| |
| Properties | |
| Molecular formula |
C3H5BrO |
| Molar mass | 136.98 g·mol−1 |
| Appearance | Colorless lachrymator |
| Density | 1.634 g/cm³ |
| Melting point | −36.5 °C (−33.7 °F; 236.7 K) |
| Boiling point | 137 °C (279 °F; 410 K) |
| Vapor pressure | 1.1 kPa (20 °C) |
| Hazards | |
| MSDS | MSDS at ILO |
| Flash point | 51.1 °C (124.0 °F; 324.2 K) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Bromoacetone is an organic compound with the formula CH3COCH2Br. This colorless liquid is a lachrymatory agent. It is a precursor to other organic compounds.
Occurrence
Bromoacetone is naturally present (less than 1%) in the essential oil of a seaweed from the vicinity of the Hawaiian Islands.[2]
Synthesis
Bromoacetone is available commercially, sometimes stabilized with magnesium oxide. It was first described in the 19th century, attributed to N. Sokolowsky.[3]

Bromoacetone is prepared by combining bromine and acetone,[4] with catalytic acid. If you use a base you will obtain bromoform instead. CH3C(O)CH3 + Br2 → CH3C(O)CH2Br + HBr The main difficulty with this method is over-bromination, resulting in di- and tribrominated products. In terms of mechanism, as with all ketones, acetone enolizes in the presence of acids or bases. The alpha carbon then undergoes electrophilic substitution with bromine.[5]
Applications
It was used in World War I as a chemical weapon, called BA by British and B-Stoff (white cross) by Germans. Due to its toxicity, it is obsolete as a riot control agent and is not used anymore. Bromoacetone is a versatile reagent in organic synthesis. It is, for example, the precursor to hydroxyacetone (b.p. 40–43°/12 mm, CAS #116-09-6).[6]
See also
- Use of poison gas in World War I
References
- ↑ Merck Index, 11th Edition, 1389
- ↑ Burreson, B. J.; Moore, R. E.; Roller, P. P. (1976). "Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta)". Journal of Agricultural and Food Chemistry 24 (4): 856–861. doi:10.1021/jf60206a040.
- ↑ Wagner, G. (1876). "Sitzung der russischen chemischen Gesellschaft am 7./19. October 1876". Berichte der Deutschen Chemischen Gesellschaft 9 (2): 1687–1688. doi:10.1002/cber.187600902196.
- ↑ Levene, P. A. (1930). "Bromoacetone". Org. Synth. 10: 12.; Coll. Vol. 2, p. 88
- ↑ Reusch, W. (2013-05-05). "Carbonyl Reactivity". Virtual Textbook of Organic Chemistry. Michigan State University.
- ↑ Levene, P. A.; Walti, A. (1930). "Acetol". Org. Synth. 10: 1.; Coll. Vol. 2, p. 5