Branched-chain amino acid
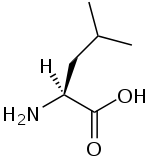
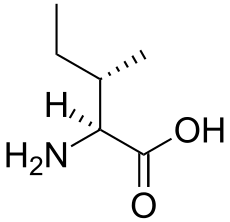
A branched-chain amino acid (BCAA) is an amino acid having aliphatic side-chains with a branch (a central carbon atom bound to three or more carbon atoms). Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine and valine.[1] Non-proteinogenic BCAAs include norvaline and 2-aminoisobutyric acid.
The three proteinogenic BCAAs are among the nine essential amino acids for humans, accounting for 35% of the essential amino acids in muscle proteins and 40% of the preformed amino acids required by mammals.[2]
Research
Dietary BCAA supplementation has been used clinically to aid in the recovery of burn victims. A 2006 paper suggests that the concept of nutrition supplemented with all BCAAs for burns, trauma, and sepsis should be abandoned for a more promising leucine-only-supplemented nutrition that requires further evaluation. [3]
Dietary BCAAs have been used in an attempt to treat some cases of hepatic encephalopathy,[4] but BCAA provides no benefit for this condition.[5]
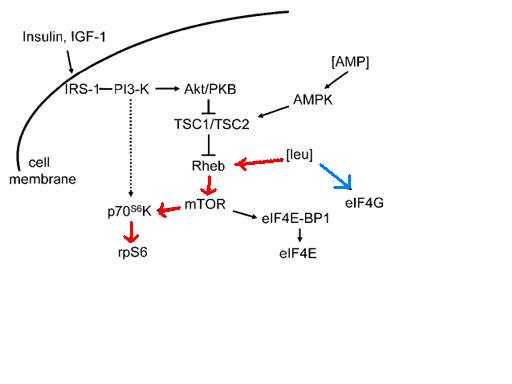
Cota et al.[6] demonstrated in 2006 that BCAAs, particularly leucine, also affect the mTOR pathway in rats, signaling two regions of their brains.
Degradation
Degradation of branched-chain amino acids involves the branched-chain alpha-keto acid dehydrogenase complex (BCKDH). A deficiency of this complex leads to a buildup of the branched-chain amino acids (leucine, isoleucine, and valine) and their toxic by-products in the blood and urine, giving the condition the name maple syrup urine disease.
The BCKDH complex converts branched-chain amino acids into Acyl-CoA derivatives, which after subsequent reactions are converted either into acetyl-CoA or succinyl-CoA that enter the citric acid cycle.[7]
Enzymes involved are branched chain aminotransferase and 3-methyl-2-oxobutanoate dehydrogenase.
See also
References
- ↑ Sowers, Strakie. "A Primer On Branched Chain Amino Acids" (PDF). Huntington College of Health Sciences. Retrieved 22 March 2011.
- ↑ Shimomura Y, Murakami T, Naoya Nakai N, Nagasaki M, Harris RA (2004). "Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise". J. Nutr. 134 (6): 1583S–1587S. Retrieved 22 March 2011.
- ↑ De Bandt JP; Cynober L (2006). "Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis". J. Nutr. 1 Suppl 136 (30): 8S–13S. Retrieved 22 March 2011.
- ↑ Chadalavada R, Sappati Biyyani RS, Maxwell J, Mullen K. (2010). "Nutrition in hepatic encephalopathy". Nutr Clin Pract. 25 (3): 257–64. doi:10.1177/0884533610368712.
- ↑ Als-Nielsen B, Koretz RL, Kjaergard LL, Gluud C (2003). "Branched-chain amino acids for hepatic encephalopathy". Cochrane Database Syst Rev (Systematic review) (2): CD001939. doi:10.1002/14651858.CD001939. PMID 12804416.
- ↑ Abstract on sciencemag.org
- ↑ Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, Chapman J, Subramaniam S (2009). "Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization". Proc. Nat. Acad. Sci. USA 106 (44): 18745–18750. doi:10.1073/pnas.0903032106. Retrieved 22 March 2011.
Further reading
- Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E (2004). "Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise". Am. J. Physiol. Endocrinol. Metab. 287 (1): E1–7. doi:10.1152/ajpendo.00430.2003. PMID 14998784.
- Blomstrand E, Eliasson J, Karlsson HK, Köhnke R (2006). "Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise". J. Nutr. 136 (1 Suppl): 269S–73S. PMID 16365096.
- Norton LE, Layman DK (2006). "Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise". J. Nutr. 136 (2): 533S–537S. PMID 16424142.
External links
- Branched-chain amino acids at the US National Library of Medicine Medical Subject Headings (MeSH)
- branched-chain amino acid degradation pathway
| ||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
.svg.png)