Boudouard reaction
The Boudouard reaction, named after Octave Leopold Boudouard, is the redox reaction of a chemical equilibrium mixture of carbon monoxide and carbon dioxide at a given temperature. It is the disproportionation of carbon monoxide into carbon dioxide and graphite or its reverse:[1]
- 2CO
 CO2 + C
CO2 + C
- 2CO
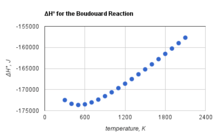
The Boudouard Reaction to form carbon dioxide and carbon is exothermic at all temperatures. However, the standard enthalpy of the Boudouard reaction becomes less negative with increasing temperature,[2] as shown to the side.
While the formation enthalpy of CO
2 is higher than that of CO, the formation entropy is much lower. Consequently, the standard free energy of formation of CO
2 from its component elements is almost constant and independent of the temperature, while the free energy of formation of CO decreases with temperature.[3] At high temperatures, the forward reaction is therefore endergonic, favoring the (exergonic) reverse reaction toward CO, even though the forward reaction is still exothermic.
The effect of temperature on the extent of the Boudouard reaction is indicated better by the value of the equilibrium constant than by the standard free energy of reaction. The value of log(Keq) for the reaction (valid between 500–2,200 K) is:[2]
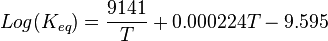
Log(Keq) has a value of zero at 975 K.
The implication of the change in Keq with temperature is that a gas containing CO and CO
2 may form elemental carbon if the mixture cools below a certain temperature. The thermodynamic activity of carbon may be calculated for a CO/CO
2 mixture by knowing the partial pressure of each species and the value of Keq. For instance, in a high temperature reducing environment, such as that created for the reduction of iron oxide in a blast furnace or the preparation of carburizing atmospheres,[4] carbon monoxide is the stable oxide of carbon. When a gas rich in CO is cooled to the point where the activity of carbon exceeds one, the Boudouard Reaction can take place. Carbon monoxide then tends to disproportionate into carbon dioxide and graphite, which forms soot.
In industrial catalysis, this is not just an eyesore; sooting (also called coking) can cause serious and even irreversible damage to catalysts and catalyst beds. This is a problem in the catalytic reforming of petroleum and the steam reforming of natural gas.
The reaction is named after the French chemist, Octave Leopold Boudouard (1872–1923), who investigated this equilibrium in 1905.[5]
Uses
Although the damaging effect of carbon monoxide on catalysts is undesirable, this reaction has been used in producing graphite flakes, filamentous graphite and lamellar graphite crystallites, as well as producing carbon nanotubes.[6][7][8][9] In graphite production, catalysts used are molybdenum, magnesium, nickel, iron and cobalt,[6][7] while in carbon nanotube production, molybdenum, nickel, cobalt, iron and Ni-MgO catalysts are used.[8][9]
The Boudouard reaction is an important process inside a blast furnace. The reduction of iron oxides is not achieved by carbon directly, as reactions between solids are typically very slow, but by carbon monoxide. The resulting carbon dioxide undergoes a Boudouard reaction upon contact with coke carbon.
References
- ↑ Bioenergylist.org – Boudouard Reaction spreadsheet
- ↑ 2.0 2.1 Reaction Web
- ↑ List of standard Gibbs free energies of formation
- ↑ ASM Committee on Furnace Atmospheres, Furnace atmospheres and carbon control, Metals Park, OH [1964].
- ↑ Holleman, Arnold F.; Wiber, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Academic Press. p. 810. ISBN 978-0-12-352651-9. Retrieved 12 July 2013.
- ↑ 6.0 6.1 Baird, T.; Fryer, J. R.; Grant, B. (Oct 1974). "Carbon" 12. pp. 591–602. doi:10.1016/0008-6223(74)90060-8.
|chapter=ignored (help) - ↑ 7.0 7.1 Trimm, D. L. (1977). "Catalysis Reviews: Science and Engineering" 16. pp. 155–189. doi:10.1080/03602457708079636.
|chapter=ignored (help) - ↑ 8.0 8.1 Dal, H. J.; Rinzler, A. G.; Nikolaev, P.; Thess, A.; Colbert, D. T.; Smalley, R. E. (1996). "Chem. Phys. Lett." 260. pp. 471–475.
|chapter=ignored (help) - ↑ 9.0 9.1 Chen, P.; Zhang, H. B.; Lin, G. D.; Hong, Q.; Tsai, K. R. (1997). "Carbon" 35. pp. 1495–1501. doi:10.1016/S0008-6223(97)00100-0.
|chapter=ignored (help)
External links
Robinson, R. J. "Boudouard Process for Synthesis Gas". ABC of Alternative Energy. Retrieved 12 July 2013.