Borophene
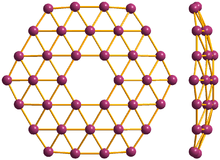
36° borophene, front and side view
Borophene is a proposed crystalline allotrope of boron. One unit consists of 36 atoms arranged in an 2-dimensional sheet with a hexagonal hole in the middle.[1][2]
Theory
Computational studies suggested that extended borophene sheets with partially filled hexagonal holes are stable. [3][4] Global minimum searches for B−
36 lead to a quasiplanar structure with a central hexagonal hole. Borophene is predicted to be fully metallic.[1]
Borophene is analogous to graphene in that it is expected to form extended sheets. The latter is a semi-metal, implying that borophene may be a better conductor.[5] The boron-boron bond is also nearly as strong as graphene’s carbon-carbon bond.[1]
Boron is adjacent to carbon in the periodic table and has similar valence orbitals. Unlike carbon, boron cannot form a honeycomb hexagonal framework (like graphene) because of its electron deficiency.[1]
History
In 2014 a research team at Brown University, led by Lai-Sheng Wang, showed that the structure of B
36 was not only possible but highly stable.[5][6][2] Photoelectron spectroscopy revealed a relatively simple spectrum, suggesting a symmetric cluster. Neutral B
36 is the smallest boron cluster to have sixfold symmetry and a perfect hexagonal vacancy, and it can be viewed as a potential basis for extended two-dimensional boron sheets.[1]
Borospherene
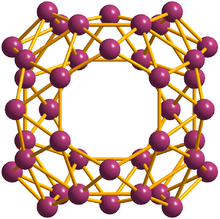
40° borospherene
In July 2014 the same team announced the creation of a 40-atom buckyball-like spherical cage made of boron that the team dubbed borospherene (derived from the original "buckminster fullerene".) Where buckyball molecules feature 20 hexagons and 12 pentagons of carbon atoms producing a smooth spherical surface, borospherene consists of 48 triangles, four seven-sided rings and two six-sided rings. The resulting shape is also spherical, but with several atoms sticking out from the sides.[7][8]
See also
| Look up borophene in Wiktionary, the free dictionary. |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Will ‘borophene’ replace graphene as a better conductor of electrons?". KurzweilAI. February 5, 2014. Retrieved February 5, 2014.
- ↑ 2.0 2.1 Piazza, Z. A.; Hu, H. S.; Li, W. L.; Zhao, Y. F.; Li, J.; Wang, L. S. (2014). "Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets". Nature Communications 5. Bibcode:2014NatCo...5E3113P. doi:10.1038/ncomms4113. PMID 24445427.
- ↑ Tang, Hui and Ismail-Beigi, Sohrab (2007). "Novel Precursors for Boron Nanotubes: The Competition of Two-Center and Three-Center Bonding in Boron Sheets". Physical Review Letters. doi:10.1103/PhysRevLett.99.115501.
- ↑ Gonzalez Szwacki, N. (2008). "Boron Fullerenes: A First-Principles Study". Nanoscale Research Letters. doi:10.1007/s11671-007-9113-1.
- ↑ 5.0 5.1 "New boron nanomaterial may be possible". Brown University. January 27, 2014. Retrieved March 9, 2013.
- ↑ Johnson, Dexter (January 28, 2014). "‘Borophene’ Might Be Joining Graphene in the 2-D Material Club". IEEE Spectrum. Retrieved March 9, 2013.
- ↑ Quick, Darren (July 14, 2014). "Borospherene bounces into buckyball family". Gizmag.com. Retrieved 2014-07-14.
- ↑ Zhai, Hua-Jin et al. (2014). "Observation of an all-boron fullerene". Nature Chemistry. doi:10.1038/nchem.1999.