Boron monofluoride
| Names | |
|---|---|
| Other names
Boron fluoride Boron(I) fluoride | |
| Identifiers | |
| 13768-60-0 | |
| ChemSpider | 4891729 |
| EC number | 237-383-0 |
| |
| Jmol-3D images | Image |
| PubChem | 6336604 |
| |
| Properties | |
| Molecular formula |
BF |
| Molar mass | 29.81 g·mol−1 |
| Thermochemistry | |
| Std molar entropy (S |
200.48 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
115.90 kJ mol−1 |
| Related compounds | |
| Related compounds |
aluminium monofluoride aluminium monochloride aluminium monoiodide gallium monofluoride |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Boron monofluoride or fluoroborylene is a chemical compound with formula BF, one atom of boron and one of fluorine. It was discovered as an unstable gas and only in 2009 found to be a stable ligand combining with transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. BF is isoelectronic with carbon monoxide and dinitrogen.
Structure
The experimental B–F bond length is 1.263 Å.[1] One reported computed bond order for the molecule is 1.4.[2]
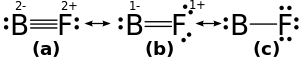
Preparation
Boron monofluoride can be prepared by passing boron trifluoride gas at 2000 °C over a boron rod. It can be condensed at liquid nitrogen temperatures (−196 °C).[3]
Reactions
BF can react with itself to form polymers of boron containing fluorine with between 10 and 14 boron atoms. BF reacts with BF3 to form B2F4. BF and B2F4 further combine to form B3F5. B3F5 is unstable above −50 °C and forms B8F12. This substance is a yellow oil.[3]
BF reacts with acetylenes to make the 1,4-diboracyclohexadiene ring system. BF can condense with 2-butyne forming 1,4-difluoro-2,3,5,6-tetramethyl-1,4-diboracyclohexadiene. Also, it reacts with acetylene to make 1,4-difluoro-1,4-diboracyclohexadiene.[3]
Ligand
The first case of BF being a ligand on a transition element was demonstrated in 2009 with the compound (C5H5)2Ru2(CO)4(μ-BF). The BF was bound to both ruthenium atoms as a bridge.[4]
Vidovic and Aldridge reacted NaRu(CO)2(C5H5) with (Et2O)·BF3.[5] Note that the BF was formed in place rather than added on.
Earlier in 1968, K. Kämpfer, H. Nöth, W. Petz, and G. Schmid claimed that Fe(BF)(CO)4 was formed in the reaction of B2F4 with Fe(CO)5, however this has not been reproduced.[5]
References
- ↑ Cazzoli, G.; Cludi, L.; Degli Esposti, C.; Dore, L. (1989). "The millimeter and submillimeter-wave spectrum of boron monofluoride: Equilibrium structure". Journal of Molecular Spectroscopy 134 (1): 159–167. Bibcode:1989JMoSp.134..159C. doi:10.1016/0022-2852(89)90138-0. ISSN 0022-2852.
- ↑ Martinie, R. J.; Bultema, J. J.; van der Wal, M. N.; Burkhart, B. J.; van der Griend, D. A.; de Kock, R. L. (2011). "Bond Order and Chemical Properties of BF, CO, and N2". Journal of Chemical Education 88 (8): 1094–1097. doi:10.1021/ed100758t.
- ↑ 3.0 3.1 3.2 Timms, P. L. (1972). "Low Temperature Condensation". Advances in Inorganic Chemistry and Radiochemistry. p. 143. ISBN 0-12-023614-1.
- ↑ Xu, L.; Li, Q.; Xie, Y.; King , R. B.; Schaefer, H. F. (2010). "Prospects for Making Organometallic Compounds with BF Ligands: Fluoroborylene Iron Carbonyls". Inorganic Chemistry 49 (3): 1046–1055. doi:10.1021/ic901964f. PMID 20041690.
- ↑ 5.0 5.1 Xu, L.; Li, Q.-S.; Xie, Y.; King, R. B.; Schaefer, H. F. III (2010). "Binuclear fluoroborylene manganese carbonyls". Inorganica Chimica Acta 363 (13): 3538–3549. doi:10.1016/j.ica.2010.07.013.
| ||||||