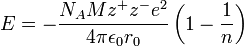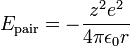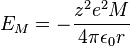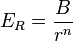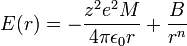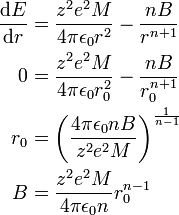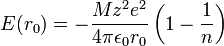Born–Landé equation
The Born–Landé equation is a means of calculating the lattice energy of a crystalline ionic compound. In 1918[1] Max Born and Alfred Landé proposed that the lattice energy could be derived from the electrostatic potential of the ionic lattice and a repulsive potential energy term.[2]
where:
- NA = Avogadro constant;
- M = Madelung constant, relating to the geometry of the crystal;
- z+ = charge number of cation
- z− = charge number of anion
- e = elementary charge, 1.6022×10−19 C
- ε0 = permittivity of free space
- 4πε0 = 1.112×10−10 C2/(J·m)
- r0 = distance to closest ion
- n = Born exponent, typically a number between 5 and 12, determined experimentally by measuring the compressibility of the solid, or derived theoretically.[3]
Derivation
The ionic lattice is modeled as an assembly of hard elastic spheres which are compressed together by the mutual attraction of the electrostatic charges on the ions. They achieve the observed equilibrium distance apart due to a balancing short range repulsion.
Electrostatic potential
The electrostatic potential energy,  , between a pair of ions of equal and opposite charge is:
, between a pair of ions of equal and opposite charge is:
where
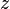 = magnitude of charge on one ion
= magnitude of charge on one ion = elementary charge, 1.6022×10−19 C
= elementary charge, 1.6022×10−19 C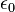 = permittivity of free space
= permittivity of free space
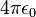 = 1.112×10−10 C²/(J m)
= 1.112×10−10 C²/(J m)
 = distance separating the ion centers
= distance separating the ion centers
For a simple lattice consisting ions with equal and opposite charge in a 1:1 ratio, interactions between one ion and all other lattice ions need to be summed to calculate 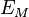 , sometimes called the Madelung or lattice energy:
, sometimes called the Madelung or lattice energy:
where
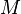 = Madelung constant, which is related to the geometry of the crystal
= Madelung constant, which is related to the geometry of the crystal = closest distance between two ions of opposite charge
= closest distance between two ions of opposite charge
Repulsive term
Born and Lande suggested that a repulsive interaction between the lattice ions would be proportional to 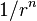 so that the repulsive energy term,
so that the repulsive energy term, 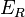 , would be expressed:
, would be expressed:
where
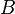 = constant scaling the strength of the repulsive interaction
= constant scaling the strength of the repulsive interaction = closest distance between two ions of opposite charge
= closest distance between two ions of opposite charge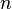 = Born exponent, a number between 5 and 12 expressing the steepness of the repulsive barrier
= Born exponent, a number between 5 and 12 expressing the steepness of the repulsive barrier
Total energy
The total intensive potential energy of an ion in the lattice can therefore be expressed as the sum of the Madelung and repulsive potentials:
Minimizing this energy with respect to  yields the equilibrium separation
yields the equilibrium separation 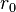 in terms of the unknown constant
in terms of the unknown constant 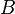 :
:
Evaluating the minimum intensive potential energy and substituting the expression for 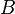 in terms of
in terms of 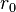 yields the Born–Landé equation:
yields the Born–Landé equation:
Calculated lattice energies
The Born–Landé equation gives a reasonable fit to the lattice energy [2]
| Compound | Calculated Lattice Energy | Experimental Lattice Energy |
|---|---|---|
| NaCl | −756 kJ/mol | −787 kJ/mol |
| LiF | −1007 kJ/mol | −1046 kJ/mol |
| CaCl2 | −2170 kJ/mol | −2255 kJ/mol |
Born Exponent
The Born exponent is typically between 5 and 12. Approximate experimental values are listed below:[4]
| Ion configuration | He | Ne | Ar, Cu+ | Kr, Ag+ | Xe, Au+ |
|---|---|---|---|---|---|
| n | 5 | 7 | 9 | 10 | 12 |
See also
References
- ↑ Brown, I. David (2002). The chemical bond in inorganic chemistry : the bond valence model (Reprint. ed.). New York: Oxford University Press. ISBN 0-19-850870-0.
- ↑ 2.0 2.1 Johnson, the Open University ; RSC ; edited by David (2002). Metals and chemical change (1. publ. ed.). Cambridge: Royal Society of Chemistry. ISBN 0-85404-665-8.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey (1980), Advanced Inorganic Chemistry (4th ed.), New York: Wiley, ISBN 0-471-02775-8
- ↑ "Lattice Energy".