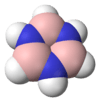
Borazine
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Borazine | |||
| Systematic IUPAC name
1,3,5,2,4,6-Triazatriborinane | |||
| Other names
borazol, inorganic benzene, borazole (after the German name for benzene, benzol) | |||
| Identifiers | |||
| 6569-51-3 | |||
| ChEBI | CHEBI:33119 | ||
| ChemSpider | 122374 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 138768 | ||
| |||
| Properties | |||
| B3H6N3 | |||
| Molar mass | 80.50 g/mol | ||
| Appearance | colourless liquid | ||
| Density | 0.81 g/cm3 | ||
| Melting point | −58 °C (−72 °F; 215 K) | ||
| Boiling point | 53 °C (127 °F; 326 K) (55 °C at 105 Pa) | ||
| Hazards | |||
| NFPA 704 | |||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Borazine is an inorganic compound with the chemical formula (BH)3(NH)3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. Like benzene, borazine is a colourless liquid.[1] For this reason borazine is sometimes referred to as "inorganic benzene".
Synthesis
The compound was reported in 1926 by the chemists Alfred Stock and Erich Pohland by a reaction of diborane with ammonia.[2]
Borazine is synthesized from diborane and ammonia in a 1:2 ratio at 250–300 °C with a conversion of 50%.
- 3 B2H6 + 6 NH3 → 2 B3H6N3 + 12 H2
An alternative more efficient route begins with lithium borohydride and ammonium chloride:
- 3 LiBH4 + 3 NH4Cl → B3H6N3 + 3 LiCl + 9 H2
In a two-step process to borazine, boron trichloride is first converted to trichloroborazine:
- 3 BCl3 + 3 NH4Cl → Cl3B3H3N3 + 9 HCl
The B-Cl bonds are subsequently converted to B-H bonds:
- 2 Cl3B3H3N3 + 6 NaBH4 → 2 B3H6N3 + 3 B2H6 + 6 NaCl
Properties
Borazine is a colourless liquid with an aromatic smell. In water it hydrolyzes to boric acid, ammonia, and hydrogen. Borazine, with a standard enthalpy change of formation ΔHf of −531 kJ/mol, is thermally very stable.
Structure and bonding
Borazine is isostructural with benzene. The six B-N bonds have length of 1.436 Å. The carbon–carbon bond in benzene is shorter length at 1.397 Å. The boron–nitrogen bond length is between that of the boron–nitrogen single bond with 0.151 nm and the boron–nitrogen double bond which is 0.131 nm. This suggests partial delocalisation of nitrogen lone-pair electrons.
The electronegativity of boron (2.04 on the Pauling scale) compared to that of nitrogen (3.04) and also the electron deficiency on the boron atom and the lone pair on nitrogen favor alternative mesomer structures for borazine.
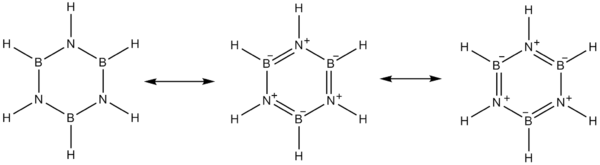
Boron is the Lewis acid and nitrogen is the Lewis base.
Reactions
Although often compared with benzene, borazine is far more reactive. With hydrogen chloride it forms an adduct, whereas benzene is unreactive toward HCl.

- B3N3H6 + 3 HCl → B3N3H9Cl3
- Addition reaction of borazine with hydrogen chloride
- B3N3H9Cl3 + NaBH4 → (BH4N)3
- reduction with sodium borohydride
The addition reaction with bromine does not require a catalyst. Borazines undergo nucleophilic attack at boron and electrophilic attack at nitrogen. Heating borazine at 70 °C expels hydrogen with formation of a borazinyl polymer or polyborazylene, in which the monomer units are coupled in a para fashion by new boron-nitrogen bonds. Boron nitride can be prepared by heating polyborazylene to 1000 °C. Borazines are also starting materials for other potential ceramics such as boron carbonitrides. Borazine can also be used as a precursor to grow boron nitride thin films on surfaces, such as the nanomesh structure which is formed on rhodium.
Polyborazylene has been proposed as a recycled hydrogen storage medium for hydrogen fuel cell vehicle applications, using a "single pot" process for digestion and reduction to recreate ammonia borane.[3]
Among other B-N type compounds mixed amino-nitro substituted borazines have been predicted to outperform carbon based explosives such as CL-20.[4]
See also
| Wikimedia Commons has media related to borazine. |
- 1,2-dihydro-1,2-azaborine — an aromatic chemical compound with properties intermediate between benzene and borazine.
References
- ↑ Duward Shriver; Peter Atkins (2010). Inorganic Chemistry (Fifth ed.). New York: W. H. Freeman and Company. p. 328. ISBN 978-1429218207.
- ↑ Stock, Alfred; Pohland, Erich (October 1926). "Borwasserstoffe, VIII. Zur Kenntnis des B2H6 und des B5H11" [Boric acid solution, VIII Regarding knowledge of B2H6 and B5H11]. Berichte (in German) 59 (9): 2210–2215. doi:10.1002/cber.19260590906.
- ↑ Davis, B. L.; Dixon, D. A.; Garner, E. B.; Gordon, J. C.; Matus, M. H.; Scott, B.; Stephens, F. H. (2009). "Efficient Regeneration of Partially Spent Ammonia Borane Fuel". Angewandte Chemie International Edition 48 (37): 6812–6816. doi:10.1002/anie.200900680. PMID 19514023.
- ↑ Koch, E.-C; Klapötke, T. M. (2012). "Boron-Based High Explosives. Propellants, Explosives, Pyrotechnics" 37. pp. 335–344. doi:10.1002/prep.201100157.
- Larry G. Sneddon; Mario G. L. Mirabelli; Anne T. Lynch; Paul J. Fazen; Kai Su; Jeffrey S. Beckdon (1991). "Polymeric precursors to boron based ceramics" (PDF). Pure & Appl. Chem. 63 (3): 407–410.
- Jong-Kyu Jeon; Yuko Uchimaru; Dong-Pyo Kim (2004). "Synthesis of Novel Amorphous Boron Carbonitride Ceramics from the Borazine Derivative Copolymer via Hydroboration". Inorg. Chem. 43 (16): 4796–4798. doi:10.1021/ic035254a.
- P. Paetzold (1991). "New perspectives in boron-nitrogen chemistry - I" (PDF). Pure & Appl. Chem. 63 (3): 345–350. doi:10.1351/pac199163030345.