Blood sugar
The blood sugar concentration or blood glucose level is the amount of glucose (sugar) present in the blood of a human or animal. The body naturally tightly regulates blood glucose levels as a part of metabolic homeostasis.
With some exceptions,[2][3] glucose is the primary source of energy for the body's cells, and blood lipids (in the form of fats and oils) are primarily a compact energy store. Glucose is transported from the intestines or liver to body cells via the bloodstream, and is made available for cell absorption via the hormone insulin, produced by the body primarily in the pancreas.
Glucose levels are usually lowest in the morning, before the first meal of the day (termed "the fasting level"), and rise after meals for an hour or two by a few millimolar. Blood sugar levels outside the normal range may be an indicator of a medical condition. A persistently high level is referred to as hyperglycemia; low levels are referred to as hypoglycemia. Diabetes mellitus is characterized by persistent hyperglycemia from any of several causes, and is the most prominent disease related to failure of blood sugar regulation. Intake of alcohol causes an initial surge in blood sugar, and later tends to cause levels to fall. Also, certain drugs can increase or decrease glucose levels.[4]
Units
The international standard way of measuring blood glucose levels are in terms of a molar concentration, measured in mmol/L (millimoles per litre; or millimolar, abbreviated mM). In the United States, West-Germany and other countries mass concentration is measured in mg/dL (milligrams per decilitre).[5]
Since the molecular weight of glucose C6H12O6 is 180, for the measurement of glucose, the difference between the two scales is a factor of 18, so that 1 mmol/L of glucose is equivalent to 18 mg/dL.[6]
Normal values in humans
Normal value ranges may vary slightly among different laboratories. Many factors affect a person's blood sugar level. A body's homeostatic mechanism, when operating normally, restores the blood sugar level to a narrow range of about 4.4 to 6.1 mmol/L (79.2 to 110 mg/dL) (as measured by a fasting blood glucose test).[7]
The normal blood glucose level (tested while fasting) for non-diabetics, should be between 3.9 and 5.5 mmol/L (70 to 100 mg/dL). The mean normal blood glucose level in humans is about 5.5 mmol/L (100 mg/dL);[6] however, this level fluctuates throughout the day. Blood sugar levels for those without diabetes and who are not fasting should be below 6.9 mmol/L (125 mg/dL).[8] The blood glucose target range for diabetics, according to the American Diabetes Association, should be 5–7.2 mmol/l (90–130 mg/dL) before meals, and less than 10 mmol/L (180 mg/dL) after meals (as measured by a blood glucose monitor).[9]
Despite widely variable intervals between meals or the occasional consumption of meals with a substantial carbohydrate load, human blood glucose levels tend to remain within the normal range. However, shortly after eating, the blood glucose level may rise, in non-diabetics, temporarily up to 7.8 mmol/L (140 mg/dL) or slightly more. For people with diabetes maintaining 'tight diabetes control', the American Diabetes Association recommends a post-meal glucose level of less than 10 mmol/L (180 mg/dL) and a fasting plasma glucose of 3.9 to 7.2 mmol/L (70–130 mg/dL).[10]
The actual amount of glucose in the blood and body fluids is very small. In a healthy adult male of 75 kg with a blood volume of 5 liters, a blood glucose level of 5.5 mmol/L (100 mg/dL) amounts to 5g, slightly less than two typical American restaurant sugar packets for coffee or tea.[11] Part of the reason why this amount is so small is that, to maintain an influx of glucose into cells, enzymes modify glucose by adding phosphate or other groups to it.
Animals
In general, ranges of blood sugar in common domestic ruminants are lower than in many monogastric mammals.[12] However this generalization does not extend to wild ruminants or camelids. For serum glucose in mg/dL, reference ranges of 42 to 75 for cows, 44 to 81 for sheep, and 48 to 76 for goats, but 61 to 124 for cats; 62 to 108 for dogs, 62 to 114 for horses, 66 to 116 for pigs, 75 to 155 for rabbits, and 90 to 140 for llamas have been reported.[13] A 90 percent reference interval for serum glucose of 26 to 181 mg/dL has been reported for captured mountain goats (Oreamnos americanus), where no effects of the pursuit and capture on measured levels were evident.[14] For beluga whales, the 25–75 percent range for serum glucose has been estimated to be 94 to 115 mg/dL.[15] For the white rhinoceros, one study has indicated that the 95 percent range is 28 to 140 mg/dL.[16] For harp seals, a serum glucose range of 4.9 to 12.1 mmol/L [i.e. 88 to 218 mg/dL] has been reported; for hooded seals, a range of 7.5 to 15.7 mmol/L [i.e. about 135 to 283 mg/dL] has been reported.[17]
Regulation
The body's homeostatic mechanism keeps blood glucose levels within a narrow range. It is composed of several interacting systems, of which hormone regulation is the most important.
There are two types of mutually antagonistic metabolic hormones affecting blood glucose levels:
- catabolic hormones (such as glucagon, cortisol and catecholamines) which increase blood glucose;
- and one anabolic hormone (insulin), which decreases blood glucose.
Abnormality in blood sugar levels
High blood sugar
If blood sugar levels remain too high the body suppresses appetite over the short term. Long-term hyperglycemia causes many of the long-term health problems including heart disease, eye, kidney, and nerve damage.
The most common cause of hyperglycemia is diabetes. When diabetes is the cause, physicians typically recommend an anti-diabetic medication as treatment. From the perspective the majority of patients, treatment with an old, well-understood diabetes drug such as metformin will be the safest, most effective, least expensive, most comfortable route to managing the condition.[18] Diet changes and exercise implementation may also be part of a treatment plan for diabetes.
Fasting blood glucose levels may be higher than the post meal blood glucose in many of the healthy subjects. Such individuals may be said to have physiological insulin resistance and may develop diabetes mellitus as long term complication. In clinical and laboratory practices, many of the time a healthy normal subject will present a fasting blood glucose value higher than the post meal blood glucose value. This creates confusion since there is a common perception that in blood, postprandial (PP) glucose level should be higher than fasting (F) glucose level. The repeated investigation subsequently yields somewhat similar type of result.[19]
Low blood sugar
If blood sugar levels drop too low, a potentially fatal condition called hypoglycemia develops. Symptoms may include lethargy, impaired mental functioning; irritability; shaking, twitching, weakness in arm and leg muscles; pale complexion; sweating; paranoid or aggressive mentality and loss of consciousness.
Mechanisms that restore satisfactory blood glucose levels after extreme hypoglycemia (below 40 mg/dl) must be quick and effective to prevent extremely serious consequences of insufficient glucose: confusion or unsteadiness and, in the extreme (below 15 mg/dl) loss of consciousness and seizures. It is far more dangerous to have too little glucose in the blood than too much, at least temporarily. In healthy individuals, blood glucose-regulating mechanisms are generally quite effective, and symptomatic hypoglycemia is generally found only in diabetics using insulin or other pharmacological treatment. Hypoglycemic episodes can vary greatly between persons and from time to time, both in severity and swiftness of onset. For severe cases, prompt medical assistance is essential, as damage to brain and other tissues and even death will result from sufficiently low blood-glucose levels.
Glucose measurement
Sample source
In a fasting individual, glucose levels are comparable in arterial, venous, and capillary blood. But following meals, capillary and arterial blood glucose levels can be significantly higher than venous levels. This is because tissue cells consume some of the glucose in the blood as it passes from arteries through the capillary bed and into the veins.[20] Although these differences vary widely, one study found that following the consumption of 50 grams of glucose, "the mean capillary blood glucose concentration is higher than the mean venous blood glucose concentration by 35%."[20][21][22]
Sample type
Glucose is measured in whole blood, plasma or serum. Historically, blood glucose values were given in terms of whole blood, but most laboratories now measure and report plasma or serum glucose levels. Because red blood cells (erythrocytes) have a higher concentration of protein (e.g., hemoglobin) than serum, serum has a higher water content and consequently more dissolved glucose than does whole blood. To convert from whole-blood glucose, multiplication by 1.15 has been shown to generally give the serum/plasma level.
Collection of blood in clot tubes for serum chemistry analysis permits the metabolism of glucose in the sample by blood cells until separated by centrifugation. Red blood cells, for instance, do not require insulin to intake glucose from the blood. Higher than normal amounts of white or red blood cell counts can lead to excessive glycolysis in the sample, with substantial reduction of glucose level if the sample is not processed quickly. Ambient temperature at which the blood sample is kept prior to centrifuging and separation of plasma/serum also affects glucose levels. At refrigerator temperatures, glucose remains relatively stable for several hours in a blood sample. Loss of glucose can be prevented by using Fluoride tubes (i.e., gray-top) since fluoride inhibits glycolysis. However, these should only be used when blood will be transported from one hospital laboratory to another for glucose measurement. Red-top serum separator tubes also preserve glucose in samples after being centrifuged isolating the serum from cells.
To prevent contamination of the sample with intravenous fluids, particular care should be given to drawing blood samples from the arm opposite the one in which an intravenous line is inserted. Alternatively, blood can be drawn from the same arm with an IV line after the IV has been turned off for at least 5 minutes, and the arm has been elevated to drain infused fluids away from the vein. Inattention can lead to large errors, since as little as 10% contamination with a 5% glucose solution (D5W) will elevate glucose in a sample by 500 mg/dL or more. Remember that the actual concentration of glucose in blood is very low, even in the hyperglycemic.
Measurement techniques
Two major methods have been used to measure glucose. The first, still in use in some places, is a chemical method exploiting the nonspecific reducing property of glucose in a reaction with an indicator substance that changes color when reduced. Since other blood compounds also have reducing properties (e.g., urea, which can be abnormally high in uremic patients), this technique can produce erroneous readings in some situations (5 to 15 mg/dL has been reported). The more recent technique, using enzymes specific to glucose, is less susceptible to this kind of error. The two most common employed enzymes are glucose oxidase and hexokinase.
In either case, the chemical system is commonly contained on a test strip which is inserted into a meter, and then has a blood sample applied. Test-strip shapes and their exact chemical composition vary between meter systems and cannot be interchanged. Formerly, some test strips were read (after timing and wiping away the blood sample) by visual comparison against a color chart printed on the vial label. Strips of this type are still used for urine glucose readings, but for blood glucose levels they are obsolete. Their error rates were, in any case, much higher. More precise blood glucose measurements are performed in a medical laboratory, using hexokinase, glucose oxidase, or glucose dehydrogenase enzymes.
Urine glucose readings, however taken, are much less useful. In properly functioning kidneys, glucose does not appear in urine until the renal threshold for glucose has been exceeded. This is substantially above any normal glucose level, and is evidence of an existing severe hyperglycemic condition. However, as urine is stored in the bladder, any glucose in it might have been produced at any time since the last time the bladder was emptied. Since metabolic conditions change rapidly, as a result of any of several factors, this is delayed news and gives no warning of a developing condition. Blood glucose monitoring is far preferable, both clinically and for home monitoring by patients. Healthy urine glucose levels were first standardized and published in 1965[23] by Hans Renschler.
| I. CHEMICAL METHODS | ||
| A. Oxidation-reduction reaction | ||
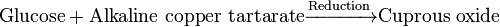 | ||
| 1. Alkaline copper reduction | ||
| Folin-Wu method |  |
Blue end-product |
|---|---|---|
| Benedict's method |
| |
| Nelson–Somogyi method |  |
Blue end-product |
| Neocuproine method | 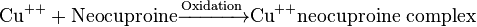 * * |
Yellow-orange color neocuproine[24] |
| Shaeffer–Hartmann–Somogyi |
| |
| 2. Alkaline Ferricyanide Reduction | ||
| Hagedorn–Jensen | 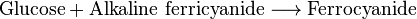 |
Colorless end product; other reducing substances interfere with reaction |
| B. Condensation | ||
| Ortho-toluidine method |
| |
| Anthrone (phenols) method |
| |
| II. ENZYMATIC METHODS | ||
| A. Glucose oxidase | ||
![\mathrm{Glucose} + \mathrm{O}_{2}\xrightarrow[\mathrm{Oxidation}] {\mathrm{glucose\ oxidase}}\textrm{D-glucono-1,5-lactone} + \mathrm{H_{2}O_{2}}](../I/m/f09986a35ceb66f76085b140cf4af542.png) | ||
| Saifer–Gerstenfeld method | ![\mathrm{H_{2}O_2} + \textit{O}\text{-dianisidine}\xrightarrow[\mathrm{Oxidation}] {\mathrm{peroxidase}} \mathrm{H_2O} + \mathrm{oxidized\ chromogen}](../I/m/b67657b74c339a36ee6da6cc731809a6.png) |
Inhibited by reducing substances like BUA, bilirubin, glutathione, ascorbic acid |
| Trinder method |
| |
| Kodak Ektachem |
| |
| Glucometer |
| |
| B. Hexokinase | ||
|
| ||
| ||
Blood glucose laboratory tests
- fasting blood sugar (i.e., glucose) test (FBS)
- two-hr postprandial blood sugar test (2-h PPBS)
- oral glucose tolerance test (OGTT)
- intravenous glucose tolerance test (IVGTT)
- glycosylated hemoglobin (HbA1C)
- self-monitoring of glucose level via patient testing
- Random blood sugar (RBS)
- Average blood glucose may be estimated by measuring glycated hemoglobin (HbA1c)
Clinical correlation
The fasting blood glucose level, which is measured after a fast of 8 hours, is the most commonly used indication of overall glucose homeostasis, largely because disturbing events such as food intake are avoided. Conditions affecting glucose levels are shown in the table below. Abnormalities in these test results are due to problems in the multiple control mechanism of glucose regulation.
The metabolic response to a carbohydrate challenge is conveniently assessed by a postprandial glucose level drawn 2 hours after a meal or a glucose load. In addition, the glucose tolerance test, consisting of several timed measurements after a standardized amount of oral glucose intake, is used to aid in the diagnosis of diabetes.
Error rates for blood glucose measurements systems vary, depending on laboratories, and on the methods used. Colorimetry techniques can be biased by color changes in test strips (from airborne or finger borne contamination, perhaps) or interference (e.g., tinting contaminants) with light source or the light sensor. Electrical techniques are less susceptible to these errors, though not to others. In home use, the most important issue is not accuracy, but trend. Thus if a meter / test strip system is consistently wrong by 10%, there will be little consequence, as long as changes (e.g., due to exercise or medication adjustments) are properly tracked. In the US, home use blood test meters must be approved by the Federal Food and Drug Administration before they can be sold.
Finally, there are several influences on blood glucose level aside from food intake. Infection, for instance, tends to change blood glucose levels, as does stress either physical or psychological. Exercise, especially if prolonged or long after the most recent meal, will have an effect as well. In the normal person, maintenance of blood glucose at near constant levels will nevertheless be quite effective.
| Persistent hyperglycemia | Transient hyperglycemia | Persistent hypoglycemia | Transient hypoglycemia |
|---|---|---|---|
| Reference range, FBG: 70–110 mg/dL | |||
| Diabetes mellitus | Pheochromocytoma | Insulinoma | Acute alcohol ingestion |
| Adrenal cortical hyperactivity Cushing's syndrome | Severe liver disease | Adrenal cortical insufficiency Addison's disease | Drugs: salicylates, antituberculosis agents |
| Hyperthyroidism | Acute stress reaction | Hypopituitarism | Severe liver disease |
| Acromegaly | Shock | Galactosemia | Several glycogen storage diseases |
| Obesity | Convulsions | Ectopic insulin production from tumors | Hereditary fructose intolerance |
Etymology and use of term
In a physiological context, the term is a misnomer because it refers to glucose, yet other sugars besides glucose are always present. Food contains several different types (e.g., fructose (largely from fruits/table sugar/industrial sweeteners), galactose (milk and dairy products), as well as several food additives such as sorbitol, xylose, maltose, etc.). But because these other sugars are largely inert with regard to the metabolic control system (i.e., that controlled by insulin secretion), since glucose is the dominant controlling signal for metabolic regulation, the term has gained currency, and is used by medical staff and lay folk alike. The table above reflects some of the more technical and closely defined terms used in the medical field.
See also
- Current research – Boronic acids in supramolecular chemistry: Saccharide recognition
- Blood glucose monitoring
- Glucagon
References
- ↑ Daly, Mark E; Vale, C; Walker, M; Littlefield, A; Alberti, KG; Mathers, JC (1998). "Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high starch diet" (PDF). Am J Clin Nutr (American Society for Clinical Nutrition) 67 (6): 1186–1196. PMID 9625092. Retrieved 2011-02-19.
- ↑ Van Soest, P. J. (1994). Nutritional ecology of the ruminant. 2nd Ed. Cornell Univ. Press, ISBN 080142772X.
- ↑ Young, J. W. (1977). "Gluconeogenesis in cattle: Significance and methodology". Journal of Dairy Science 60 (1): 1–15. doi:10.3168/jds.S0022-0302(77)83821-6. PMID 320235.
- ↑ Rosemary Walker & Jill Rodgers Type 2 Diabetes – Your Questions Answered, Dorling Kindersley, 2006, ISBN 1-74033-550-3.
- ↑ Diabetes FAQs – Blood Glucose Measurement Units – Abbott Diabetes Care
- ↑ 6.0 6.1 What are mg/dl and mmol/l? How to convert? Glucose? Cholesterol? Advameg, Inc.
- ↑ "Screening for Type 2 Diabetes". Clinical Diabetes 18 (2). 2000.
- ↑ Glucose test – blood. NIH – National Institutes of Health.
- ↑ Davidson, Nancy Klobassa and Moreland, Peggy (July 26, 2011) Living with diabetes blog. mayoclinic.com.
- ↑ American Diabetes Association (2006). "January 2006 Diabetes Care". Diabetes Care 29 (Supplement 1): 51–580. PMID 16373931.
Standards of Medical Care-Table 6 and Table 7, Correlation between A1C level and Mean Plasma Glucose Levels on Multiple Testing over 2–3 months
- ↑ USDA National Nutrient Database for Standard Reference, Release 22 (2009)
- ↑ Eiler, H. (2004). "Endocrine glands". In Reese, W. O. Dukes' Physiology of Domestic Animals (12th ed.). Ithaca, NY: Comstock. pp. 621–669. ISBN 0801442389.
- ↑ Kahn, C. M., ed. (2005). Merck Veterinary Manual (9th ed.). Whitehouse Station: Merck & Co. ISBN 0911910506.
- ↑ Rice, C. G. and Hall, B. (2007). "Hematologic and biochemical reference intervals for mountain goats (Oreamnos americanus): effects of capture conditions". Northwest Science 81 (3): 206. doi:10.3955/0029-344X-81.3.206.
- ↑ Cornell, LH; Duffield, DS; Joseph, BE; Stark, B (1988). "Hematology and serum chemistry values in the beluga (Delphinapterus leucas)". Journal of wildlife diseases 24 (2): 220–4. doi:10.7589/0090-3558-24.2.220. PMID 3373628.
- ↑ Seal, U. S.; Barton, R.; Mather, L.; Gray, C. W. (1976). "Baseline Laboratory Data for the White Rhinoceros (Ceratotherium simum simum)" (PDF). The Journal of Zoo Animal Medicine 7 (1): 11–17. JSTOR 20094341.
- ↑ Boily, F; Beaudoin, S; Measures, LN (2006). "Hematology and serum chemistry of harp (Phoca groenlandica) and hooded seals (Cystophora cristata) during the breeding season, in the Gulf of St. Lawrence, Canada". Journal of wildlife diseases 42 (1): 115–32. doi:10.7589/0090-3558-42.1.115. PMID 16699154.
- ↑ Consumer Reports Health Best Buy Drugs. "The Oral Diabetes Drugs: Treating Type 2 Diabetes" (PDF). Best Buy Drugs (Consumer Reports): 2. Retrieved September 18, 2012.
- ↑ Warade, Jayesh Prabhakar (2014). "Fasting blood glucose level higher than post-meal in healthy subjects: a study of 738 subjects" (PDF). World Journal of Pharmaceutical Research 3 (4).
- ↑ 20.0 20.1 Yang, Chiaohsin (2012). "A Comparison between Venous and Finger-Prick Blood Sampling on Values of Blood Glucose" (PDF). International Proceedings of Chemical, Biological and Environmental Engineering 39: 236.
- ↑ Somogyi, Michael (1948). "Studies of Arteriovenous Differences in Blood Sugar" (PDF). J. Biol. Chem 174 (1): 189–200. PMID 18914074.
- ↑ Roe, Jeffrey. "Glucose Concentration Difference Between Arterial, Capillary, and Venous Blood."
- ↑ Renschler, HE; Weicker, H; Von Baeyer, H (1965). "The upper limit of glucose concentration in the urine of healthy subjects". Deutsche Medizinische Wochenschrift 90 (53): 2349–53. PMID 5851934.
- ↑ Neocuproine MSDS. hazard.com
Further reading
- Henry, John Bernard (2001). Clinical diagnosis and Management by Laboratory Methods (20th ed.). Philadelphia: Saunders. ISBN 0721688640.
External links
- Glucose at Lab Tests Online
- Glucose (blood, serum, plasma): analyte monograph – The Association for Clinical Biochemistry and Laboratory Medicine
| ||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

![\begin{alignat}{2}
& \mathrm{Glucose} + \mathrm{ATP}\xrightarrow[\mathrm{Phosphorylation}] {\mathrm{Hexokinase} + \mathrm{Mg}^{++}} \textrm{G-6PO}_4 + \mathrm{ADP} \\
& \textrm{G-6PO}_4 + \mathrm{NADP}\xrightarrow[\mathrm{Oxidation}] {\textrm{G-6PD}} \textrm{6-Phosphogluconate} + \mathrm{NADPH} + \mathrm{H}^{+} \\
\end{alignat}](../I/m/76a16563d98e3cda70668458df7f3602.png)