Bisphenol S
 | |
| Names | |
|---|---|
| IUPAC name
4,4'-Sulfonyldiphenol | |
| Other names
BPS, 4,4'-sulfonylbisphenol, bis(4-hydroxyphenyl)sulfone | |
| Identifiers | |
| 80-09-1 | |
| ChEMBL | ChEMBL384441 |
| ChemSpider | 6374 |
| |
| Jmol-3D images | Image Image |
| KEGG | C14216 |
| PubChem | 6626 |
| |
| Properties | |
| Molecular formula |
C12H10O4S |
| Molar mass | 250.27 g·mol−1 |
| Appearance | White colorless solid; forms needle shaped crystals in water |
| Density | 1.3663 g/cm3 |
| Melting point | 245 to 250 °C (473 to 482 °F; 518 to 523 K)[1] |
| insoluble | |
| Solubility | soluble in ethanol |
| Hazards | |
| R-phrases | R36 |
| S-phrases | S26 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
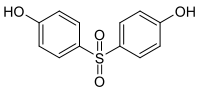
Bisphenol S (BPS) is an organic compound with the formula (HOC6H4)2SO2. It has two phenol functional groups on either side of a sulfonyl group. It is commonly used in curing fast-drying epoxy resin adhesives. It is an analog of bisphenol A (BPA) in which the dimethylmethylene group (C(CH3)2) is replaced with a sulfone group (SO2).
Use
BPS is used in curing fast-drying epoxy glues and as an corrosion inhibitor. It is also commonly used as a reactant in polymer reactions.
BPS has become increasingly common as a plasticizing agent following the widespread bans on the use of BPA due to its estrogen-mimicking properties, and BPS can now be found in a variety of common consumer products.[2][3] In some cases, BPS is used where the legal prohibition on BPA allows products containing BPS to be labelled "BPA free".[4] BPS also has the advantage of being more stable to heat and light than BPA.[5]
To comply with restrictions and regulations on BPA due to it's confirmed toxicity, manufacturers are gradually replacing BPA with other related compounds, mainly bisphenol S (BPS; 4,4′-sulfonyldiphenol), as substitutes in industrial applications. [6]
BPS is also used as an anticorrosive agent in epoxy glues. Chemically, BPS is being used as a reagent in polymer reactions. BPS has also been reported to occur in canned foodstuffs. [7]
In a recent study analyzing BPS in a variety of paper products worldwide, BPS was found in 100% of tickets, mailing envelopes, airplane boarding passes, and airplane luggage tags. In this study, very high concentrations of BPS were detected in thermal receipt samples collected from cities in the United States, Japan, Korea, and Vietnam. The BPS concentrations were large but varied greatly, from a few tens of nanograms per gram to several milligrams per gram, [8]
Toxicity
BPS has been shown to have similar in vitro estrogenic activity to BPA.[5][9][10] One study showed that exposure to low levels of BPS in cultured rat pituitary cells altered the estrogen estradiol signaling pathway to induce inappropriate release of prolactin, which, as a result, affected cell proliferation and apoptosis.[11]
BPS has also been linked to changes in neurodevelopment. In a 2014 study performed by researchers at the University of Calgary, exposure to low levels of BPS disrupted the timing of neurogenesis within the hypothalamus in embryonic zebrafish. The rate at which neurons developed increased by 240 percent. [12]
The recycling of thermal paper can introduce BPS into the cycle of paper production and cause BPS contamination of other types of paper products.[13][14]
Though BPA was proven harmful to the environment and fairly resistant to environmental degradation, Japanese researchers showed that some BPA substitutes, including BPS, were more resistant to environmental degradation than BPA. [15]
History
BPS was first made in 1869 as a dye.[16]
Studies have shown that BPS is not a chemical that is newly incorporated into everyday consumer products, but one that is just being brought to public awareness.[17]
Regulation
It is difficult for consumers to determine if a product contains BPS due to limited labeling regulations.[18]
Synthesis
Bisphenol S is prepared by the reaction of two equivalents of phenol with one equivalent of sulfuric acid or oleum.[19]
- 2 C6H5OH + H2SO4 → (C6H4OH)2SO2 + 2 H2O
- 2 C6H5OH + SO3 → (C6H4OH)2SO2 + H2O
This reaction can also produce 2,4'-sulfonyldiphenol, a common isomeric complication in electrophilic aromatic substitution reactions.
See also
References
- ↑ www.sigmaaldrich.com
- ↑ Liao, C.; Liu, F.; Kannan, K. (2012). "Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol a Residues". Environmental Science & Technology 46 (12): 6515–22. doi:10.1021/es300876n. PMID 22591511.
- ↑ Liao, C.; Liu, F.; Guo, Y.; Moon, H. B.; Nakata, H.; Wu, Q.; Kannan, K. (2012). "Occurrence of Eight Bisphenol Analogues in Indoor Dust from the United States and Several Asian Countries: Implications for Human Exposure". Environmental Science & Technology 46 (16): 9138–9145. doi:10.1021/es302004w.
- ↑ Jenna Bilbrey (Aug 11, 2014). "BPA-Free Plastic Containers May Be Just as Hazardous". Scientific American.
- ↑ 5.0 5.1 Kuruto-Niwa, R.; Nozawa, R.; Miyakoshi, T.; Shiozawa, T.; Terao, Y. (2005). "Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system". Environmental Toxicology and Pharmacology 19 (1): 121–130. doi:10.1016/j.etap.2004.05.009. PMID 21783468.
- ↑ Chen, M. Y.; Ike, M.; Fujita, M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ. Toxicol. 2002, 17 (1), 80−86.
- ↑ (Viñas, P.; Campillo, N.; Martínez-Castillo, N.; Hernandez- ́ Cordoba, M. Comparison of two derivatization-based methods for ́ solid-phase microextraction-gas chromatography-mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Anal. Bioanal. Chem. 2010, 397 (1), 115−125.)
- ↑ Liao, Chunyang, Fang Liu, and Kurunthachalam Kannan. "Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol A residues." Environmental science & technology 46.12 (2012): 6515-6522.
- ↑ Viñas, R.; Watson, C. S. (2013). "Bisphenol S Disrupts Estradiol-Induced Nongenomic Signaling in a Rat Pituitary Cell Line: Effects on Cell Functions". Environmental Health Perspectives 121 (3): 352–8. doi:10.1289/ehp.1205826. PMID 23458715.
- ↑ Ji, K.; Hong, S.; Kho, Y.; Choi, K. (2013). "Effects of Bisphenol S Exposure on Endocrine Functions and Reproduction of Zebrafish". Environmental Science & Technology: 8793–8800. doi:10.1021/es400329t.
- ↑ Viñas, R.; Watson, C. S. (2013). "Bisphenol S Disrupts Estradiol-Induced Nongenomic Signaling in a Rat Pituitary Cell Line: Effects on Cell Functions". Environmental Health Perspectives 121 (3): 352–8. doi:10.1289/ehp.1205826. PMID 23458715.
- ↑ Kinch, Cassandra D et al. "Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish". PNAS. Proceedings of the National Academy of Sciences of the United States of America. PMID 25583509.
- ↑ Gehring, M.; Tennhardt, L.; Vogel, D.; Weltin, D.; Bilitewski, B. Bisphenol A Contamination of Wastepaper, Cellulose and Recycled Paper Products. In Waste Management and the Environment II. WIT Transactions on Ecology and the Environment; Brebbia, C. A., Kungulos, S., Popov, V., Itoh, H., Eds.; WIT Press: Southampton, Boston, 2004; Vol. 78, pp 294−300.
- ↑ European Commission-Joint Research Centre. European Union Risk Assessment Report, 4,4′-Isopropylidenediphenol (Bisphenol-A). 2008, available from http://ecb.jrc.ec.europa.eu/documents/ExistingChemicals/RISK_ASSESSMENT/ADDENDUM/bisphenola_add_ 325.pdf
- ↑ Ike, M.; Chen, M. Y.; Danzl, E.; Sei, K.; Fujita, M. Biodegradation of a variety of bisphenols under aerobic and anaerobic conditions. Water Sci. Technol. 2006, 53 (6), 153−159.
- ↑ Glausiusz, Josie. "Toxicology: The plastics puzzle". Nature Publishing Group. Retrieved 25 March 2015.
- ↑ Brian, Bienkowski. "BPA replacement alters hormones at low doses, study finds". Environmental Health News. Environmental Health Sciences. Retrieved 23 March 2015.
- ↑ Howard, Brian. "Chemical in BPA-Free Products Linked to Irregular Heartbeats". National Geographic. National Geographic. Retrieved 29 March 2015.
- ↑ METHOD OF PREPARATION OF 4,4′-DIHYDROXYDIPHENYLSULPHONE (Freepatentsonline).
