Bird migration

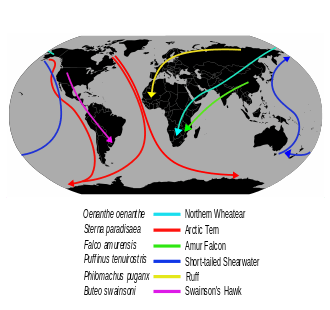
Bird migration is the regular seasonal movement, often north and south along a flyway, between breeding and wintering grounds. Many species of bird migrate. Migration carries high costs in predation and mortality, including from hunting by humans. Migration is driven primarily by availability of food. It occurs mainly in the northern hemisphere, where birds are funnelled on to specific routes by natural barriers such as the Mediterranean Sea or the Caribbean Sea.
Historically, migration has been recorded as much as 3,000 years ago by Ancient Greek authors including Homer and Aristotle, and in the Book of Job, for species such as storks, turtle doves, and swallows. More recently, Johannes Leche began recording dates of arrivals of spring migrants in Finland in 1749, and scientific studies have used techniques including bird ringing and satellite tracking. Threats to migratory birds have grown with habitat destruction especially of stopover and wintering sites, as well as structures such as power lines and wind farms.
The Arctic tern holds the long-distance migration record for birds, travelling between Arctic breeding grounds and the Antarctic each year. Some species of tubenoses (Procellariiformes) such as albatrosses circle the earth, flying over the southern oceans, while others such as Manx shearwaters migrate 14,000 km (8,700 mi) between their northern breeding grounds and the southern ocean. Shorter migrations are common, including altitudinal migrations on mountains such as the Andes and Himalayas.
The timing of migration seems to be controlled primarily by changes in day length. Migrating birds navigate using celestial cues from the sun and stars, the earth's magnetic field, and probably also mental maps. Migration has developed independently in different groups of birds and does not appear to require genetic change; some birds have acquired migratory behaviour since the last ice age.
Historical views
Records of bird migration were made as much as 3,000 years ago by the Ancient Greek writers Hesiod, Homer, Herodotus and Aristotle. The Bible also notes migrations, as in the Book of Job (39:26), where the inquiry is made: "Is it by your insight that the hawk hovers, spreads its wings southward?" The author of Jeremiah (8:7) wrote: "Even the stork in the heavens knows its seasons, and the turtle dove, the swift and the crane keep the time of their arrival."
Aristotle noted that cranes traveled from the steppes of Scythia to marshes at the headwaters of the Nile. Pliny the Elder, in his Historia Naturalis, repeats Aristotle's observations.[1]
Swallow migration versus hibernation
Aristotle however suggested that swallows and other birds hibernated. This belief persisted as late as 1878, when Elliott Coues listed the titles of no less than 182 papers dealing with the hibernation of swallows. Even the "highly observant"[2] Gilbert White, in his posthumously published 1789 The Natural History of Selborne, quoted a man's story about swallows being found in a chalk cliff collapse "while he was a schoolboy at Brighthelmstone", though the man denied being an eyewitness.[3] However, he also writes that "as to swallows being found in a torpid state during the winter in the Isle of Wight or any part of this country, I never heard any such account worth attending to",[3] and that if early swallows "happen to find frost and snow they immediately withdraw for a time—a circumstance this much more in favour of hiding than migration", since he doubts they would "return for a week or two to warmer latitudes".[4]
It was not until the end of the eighteenth century that migration as an explanation for the winter disappearance of birds from northern climes was accepted.[1] Thomas Bewick's A History of British Birds (Volume 1, 1797) mentions a report from "a very intelligent master of a vessel" who, "between the islands of Minorca and Majorca, saw great numbers of Swallows flying northward",[5] and states the situation in Britain as follows:
Swallows frequently roost at night, after they begin to congregate, by the sides of rivers and pools, from which circumstance it has been erroneously supposed that they retire into the water.—Bewick[6]
Bewick then describes an experiment which succeeded in keeping swallows alive in Britain for several years, where they remained warm and dry through the winters. He concludes:
These experiments have since been amply confirmed by ... M. Natterer, of Vienna ... and the result clearly proves, what is in fact now admitted on all hands, that Swallows do not in any material instance differ from other birds in their nature and propensities [for life in the air]; but that they leave us when this country can no longer furnish them with a supply of their proper and natural food ...—Bewick[7]
General patterns


Migration is the regular seasonal movement, often north and south, undertaken by many species of birds. Bird movements include those made in response to changes in food availability, habitat, or weather. Sometimes, journeys are not termed "true migration" because they are irregular (nomadism, invasions, irruptions) or in only one direction (dispersal, movement of young away from natal area). Migration is marked by its annual seasonality.[8] Non-migratory birds are said to be resident or sedentary. Approximately 1800 of the world's 10,000 bird species are long-distance migrants.[9][10]
Many bird populations migrate long distances along a flyway. The most common pattern involves flying north in the spring to breed in the temperate or Arctic summer and returning in the autumn to wintering grounds in warmer regions to the south. Of course, in the southern hemisphere the directions are reversed, but there is less land area in the far south to support long-distance migration.[11]
The primary motivation for migration appears to be food; for example, some hummingbirds choose not to migrate if fed through the winter.[12] Also, the longer days of the northern summer provide extended time for breeding birds to feed their young. This helps diurnal birds to produce larger clutches than related non-migratory species that remain in the tropics. As the days shorten in autumn, the birds return to warmer regions where the available food supply varies little with the season.[13]
These advantages offset the high stress, physical exertion costs, and other risks of the migration. Predation can be heightened during migration: Eleonora's falcon Falco eleonorae, which breeds on Mediterranean islands, has a very late breeding season, coordinated with the autumn passage of southbound passerine migrants, which it feeds to its young. A similar strategy is adopted by the greater noctule bat, which preys on nocturnal passerine migrants.[14][15][16] The higher concentrations of migrating birds at stopover sites make them prone to parasites and pathogens, which require a heightened immune response.[11]
Within a species not all populations may be migratory; this is known as "partial migration". Partial migration is very common in the southern continents; in Australia, 44% of non-passerine birds and 32% of passerine species are partially migratory.[17] In some species, the population at higher latitudes tends to be migratory and will often winter at lower latitude. The migrating birds bypass the latitudes where other populations may be sedentary, where suitable wintering habitats may already be occupied. This is an example of leap-frog migration.[18] Many fully migratory species show leap-frog migration (birds that nest at higher latitudes spend the winter at lower latitudes), and many show the alternative, chain migration, where populations 'slide' more evenly north and south without reversing order.[19]
Within a population, it is common for different ages and/or sexes to have different patterns of timing and distance. Female chaffinches Fringilla coelebs in Eastern Fennoscandia migrate earlier in the autumn than males do.[20]
Most migrations begin with the birds starting off in a broad front. Often, this front narrows into one or more preferred routes termed flyways. These routes typically follow mountain ranges or coastlines, sometimes rivers, and may take advantage of updrafts and other wind patterns or avoid geographical barriers such as large stretches of open water. The specific routes may be genetically programmed or learned to varying degrees. The routes taken on forward and return migration are often different.[11] A common pattern in North America is clockwise migration, where birds flying North tend to be further West, and flying South tend to shift Eastwards.
Many, if not most, birds migrate in flocks. For larger birds, flying in flocks reduces the energy cost. Geese in a V-formation may conserve 12–20% of the energy they would need to fly alone.[21][22] Red knots Calidris canutus and dunlins Calidris alpina were found in radar studies to fly 5 km/h (3.1 mph) faster in flocks than when they were flying alone.[11]

Birds fly at varying altitudes during migration. An expedition to Mt. Everest found skeletons of northern pintail Anas acuta and black-tailed godwit Limosa limosa at 5,000 m (16,000 ft) on the Khumbu Glacier.[23] Bar-headed geese Anser indicus have been recorded by GPS flying at up to 6,540 metres (21,460 ft) while crossing the Himalayas, at the same time engaging in the highest rates of climb to altitude for any bird. Anecdotal reports of them flying much higher have yet to be corroborated with any direct evidence.[24] Seabirds fly low over water but gain altitude when crossing land, and the reverse pattern is seen in landbirds.[25][26] However most bird migration is in the range of 150 to 600 m (490 to 1,970 ft). Bird strike aviation records from the United States show most collisions occur below 600 m (2,000 ft) and almost none above 1,800 m (5,900 ft).[27]
Bird migration is not limited to birds that can fly. Most species of penguin (Spheniscidae) migrate by swimming. These routes can cover over 1,000 km (620 mi). Blue grouse Dendragapus obscurus perform altitudinal migration mostly by walking. Emus Dromaius novaehollandiae in Australia have been observed to undertake long-distance movements on foot during droughts.[11]
Long-distance migration
The typical image of migration is of northern landbirds, such as swallows (Hirundinidae) and birds of prey, making long flights to the tropics. However, many Holarctic wildfowl and finch (Fringillidae) species winter in the North Temperate Zone, in regions with milder winters than their summer breeding grounds. For example, the pink-footed goose Anser brachyrhynchus migrates from Iceland to Britain and neighbouring countries, whilst the dark-eyed junco Junco hyemalis migrates from subarctic and arctic climates to the contiguous United States[28] and the American goldfinch from taiga to wintering grounds extending from the American South northwestward to Western Oregon.[29] Migratory routes and wintering grounds are traditional and learned by young during their first migration with their parents. Some ducks, such as the garganey Anas querquedula, move completely or partially into the tropics. The European pied flycatcher Ficedula hypoleuca also follows this migratory trend, breeding in Asia and Europe and wintering in Africa.
The same considerations about barriers and detours that apply to long-distance land-bird migration apply to water birds, but in reverse: a large area of land without bodies of water that offer feeding sites may also be a barrier to a bird that feeds in coastal waters. Detours avoiding such barriers are observed: for example, brent geese Branta bernicla migrating from the Taymyr Peninsula to the Wadden Sea travel via the White Sea coast and the Baltic Sea rather than directly across the Arctic Ocean and northern Scandinavia.[30]
In waders
A similar situation occurs with waders (called shorebirds in North America). Many species, such as dunlin Calidris alpina[31] and western sandpiper Calidris mauri,[32] undertake long movements from their Arctic breeding grounds to warmer locations in the same hemisphere, but others such as semipalmated sandpiper C. pusilla travel longer distances to the tropics in the Southern Hemisphere.[33]
For some species of waders, migration success depends on the availability of certain key food resources at stopover points along the migration route. This gives the migrants an opportunity to refuel for the next leg of the voyage. Some examples of important stopover locations are the Bay of Fundy and Delaware Bay.[34][35]
Some bar-tailed godwits Limosa lapponica have the longest known non-stop flight of any migrant, flying 11,000 km from Alaska to their New Zealand non-breeding areas.[36] Prior to migration, 55 percent of their bodyweight is stored as fat to fuel this uninterrupted journey.
In seabirds

Seabird migration is similar in pattern to those of the waders and waterfowl. Some, such as the black guillemot Cepphus grylle and some gulls, are quite sedentary; others, such as most terns and auks breeding in the temperate northern hemisphere, move varying distances south in the northern winter. The Arctic tern Sterna paradisaea has the longest-distance migration of any bird, and sees more daylight than any other, moving from its Arctic breeding grounds to the Antarctic non-breeding areas.[37] One Arctic tern, ringed (banded) as a chick on the Farne Islands off the British east coast, reached Melbourne, Australia in just three months from fledging, a sea journey of over 22,000 km (14,000 mi). Many tubenosed birds breed in the southern hemisphere and migrate north in the southern winter.[38]
The most pelagic species, mainly in the 'tubenose' order Procellariiformes, are great wanderers, and the albatrosses of the southern oceans may circle the globe as they ride the "roaring forties" outside the breeding season. The tubenoses spread widely over large areas of open ocean, but congregate when food becomes available. Many are also among the longest-distance migrants; sooty shearwaters Puffinus griseus nesting on the Falkland Islands migrate 14,000 km (8,700 mi) between the breeding colony and the North Atlantic Ocean off Norway. Some Manx shearwaters Puffinus puffinus do this same journey in reverse. As they are long-lived birds, they may cover enormous distances during their lives; one record-breaking Manx shearwater is calculated to have flown 8 million km (5 million miles) during its over-50 year lifespan.[39]
Diurnal migration in raptors

Some large broad-winged birds rely on thermal columns of rising hot air to enable them to soar. These include many birds of prey such as vultures, eagles, and buzzards, but also storks. These birds migrate in the daytime. Migratory species in these groups have great difficulty crossing large bodies of water, since thermals only form over land, and these birds cannot maintain active flight for long distances. Mediterranean and other seas present a major obstacle to soaring birds, which must cross at the narrowest points. Massive numbers of large raptors and storks pass through areas such as Gibraltar, Falsterbo, and the Bosphorus at migration times. More common species, such as the European honey buzzard Pernis apivorus, can be counted in hundreds of thousands in autumn. Other barriers, such as mountain ranges, can also cause funnelling, particularly of large diurnal migrants. This is a notable factor in the Central American migratory bottleneck. Batumi bottleneck in the Caucasus is one of the heaviest migratory funnels on earth. Avoiding flying over the Black Sea surface and across high mountains, hundreds of thousands of soaring birds funnel through an area around the city of Batumi, Georgia.[40] Birds of prey such as honey buzzards which migrate using thermals lose only 10 to 20% of their weight during migration, which may explain why they forage less during migration than do smaller birds of prey with more active flight such as falcons, hawks and harriers.[41]
Nocturnal migration in smaller insectivorous birds
Many of the smaller insectivorous birds including the warblers, hummingbirds and flycatchers migrate large distances, usually at night. They land in the morning and may feed for a few days before resuming their migration. The birds are referred to as passage migrants in the regions where they occur for short durations between the origin and destination.[42]
Nocturnal migrants minimize predation, avoid overheating, and can feed during the day.[1] One cost of nocturnal migration is the loss of sleep. Migrants may be able to alter their quality of sleep to compensate for the loss.[43]
Short-distance and altitudinal migration
Many long-distance migrants appear to be genetically programmed to respond to changing day length. Species that move short distances, however, may not need such a timing mechanism, instead moving in response to local weather conditions. Thus mountain and moorland breeders, such as wallcreeper Tichodroma muraria and white-throated dipper Cinclus cinclus, may move only altitudinally to escape the cold higher ground. Other species such as merlin Falco columbarius and Eurasian skylark Alauda arvensis move further, to the coast or towards the south. Species like the chaffinch are much less migratory in Britain than those of continental Europe, mostly not moving more than 5 km in their lives.[44]
Short-distance passerine migrants have two evolutionary origins. Those that have long-distance migrants in the same family, such as the common chiffchaff Phylloscopus collybita, are species of southern hemisphere origins that have progressively shortened their return migration to stay in the northern hemisphere.[45]
Species that have no long-distance migratory relatives, such as the waxwings Bombycilla, are effectively moving in response to winter weather and the loss of their usual winter food, rather than enhanced breeding opportunities.[46]
In the tropics there is little variation in the length of day throughout the year, and it is always warm enough for a food supply, but altitudinal migration occurs in some tropical birds. There is evidence that this enables the migrants to obtain more of their preferred foods such as fruits.[47]
Altitudinal migration is common on mountains worldwide, such as in the Himalayas and the Andes.[48]
Irruptions and dispersal

Sometimes circumstances such as a good breeding season followed by a food source failure the following year lead to irruptions in which large numbers of a species move far beyond the normal range. Bohemian waxwings Bombycilla garrulus well show this unpredictable variation in annual numbers, with five major arrivals in Britain during the nineteenth century, but 18 between the years 1937 and 2000.[46] Red crossbills Loxia curvirostra too are irruptive, with widespread invasions across England noted in 1251, 1593, 1757, and 1791.[49]
Bird migration is primarily, but not entirely, a Northern Hemisphere phenomenon.[50] This is because land birds in high northern latitudes, where food becomes scarce in winter, leave for areas further south (including the Southern Hemisphere) to overwinter, and because the continental landmass is much larger in the Northern Hemisphere. In contrast, among (pelagic) seabirds, species of the Southern Hemisphere are more likely to migrate. This is because there is a large area of ocean in the Southern Hemisphere, and more islands suitable for seabirds to nest.[51]
Physiology and control
The control of migration, its timing and response are genetically controlled and appear to be a primitive trait that is present even in non-migratory species of birds. The ability to navigate and orient themselves during migration is a much more complex phenomenon that may include both endogenous programs as well as learning.[52]
Timing
The primary physiological cue for migration are the changes in the day length. These changes are also related to hormonal changes in the birds. In the period before migration, many birds display higher activity or Zugunruhe (German: migratory restlessness), first described by Johann Friedrich Naumann in 1795, as well as physiological changes such as increased fat deposition. The occurrence of Zugunruhe even in cage-raised birds with no environmental cues (e.g. shortening of day and falling temperature) has pointed to the role of circannual endogenous programs in controlling bird migrations.[53] Caged birds display a preferential flight direction that corresponds with the migratory direction they would take in nature, changing their preferential direction at roughly the same time their wild conspecifics change course.[54]
In polygynous species with considerable sexual dimorphism, males tend to return earlier to the breeding sites than their females. This is termed protandry.[55][56]
Orientation and navigation
Navigation is based on a variety of senses. Many birds have been shown to use a sun compass. Using the sun for direction involves the need for making compensation based on the time. Navigation has also been shown to be based on a combination of other abilities including the ability to detect magnetic fields (magnetoception), use visual landmarks as well as olfactory cues.[57]
Long distance migrants are believed to disperse as young birds and form attachments to potential breeding sites and to favourite wintering sites. Once the site attachment is made they show high site-fidelity, visiting the same wintering sites year after year.[58]
The ability of birds to navigate during migrations cannot be fully explained by endogenous programming, even with the help of responses to environmental cues. The ability to successfully perform long-distance migrations can probably only be fully explained with an accounting for the cognitive ability of the birds to recognize habitats and form mental maps. Satellite tracking of day migrating raptors such as ospreys and honey buzzards has shown that older individuals are better at making corrections for wind drift.[59]
Migratory birds may use two electromagnetic tools to find their destinations: one that is entirely innate and another that relies on experience. A young bird on its first migration flies in the correct direction according to the Earth's magnetic field, but does not know how far the journey will be. It does this through a radical pair mechanism whereby chemical reactions in special photo pigments sensitive to long wavelengths are affected by the field. Although this only works during daylight hours, it does not use the position of the sun in any way. At this stage the bird is in the position of a boy scout with a compass but no map, until it grows accustomed to the journey and can put its other capabilities to use. With experience it learns various landmarks and this "mapping" is done by magnetites in the trigeminal system, which tell the bird how strong the field is. Because birds migrate between northern and southern regions, the magnetic field strengths at different latitudes let it interpret the radical pair mechanism more accurately and let it know when it has reached its destination.[60] There is a neural connection between the eye and "Cluster N", the part of the forebrain that is active during migrational orientation, suggesting that birds may actually be able to see the magnetic field of the earth.[61][62]
Vagrancy
Migrating birds can lose their way and appear outside their normal ranges. This can be due to flying past their destinations as in the "spring overshoot" in which birds returning to their breeding areas overshoot and end up further north than intended. Certain areas, because of their location, have become famous as watchpoints for such birds. Examples are the Point Pelee National Park in Canada, and Spurn in England.
Reverse migration, where the genetic programming of young birds fails to work properly, can lead to rarities turning up as vagrants thousands of kilometres out of range.[63]
Drift migration of birds blown off course by the wind can result in "falls" of large numbers of migrants at coastal sites.[64]
A related phenomenon called "abmigration" involves birds from one region joining similar birds from a different breeding region in the common winter grounds and then migrating back along with the new population. This is especially common in some waterfowl, which shift from one flyway to another.[65]
Migration conditioning
It has been possible to teach a migration route to a flock of birds, for example in re-introduction schemes. After a trial with Canada geese Branta canadensis, microlight aircraft were used in the US to teach safe migration routes to reintroduced whooping cranes Grus americana.[66][67]
Adaptations
Birds need to alter their metabolism in order to meet the demands of migration. The storage of energy through the accumulation of fat and the control of sleep in nocturnal migrants require special physiological adaptations. In addition, the feathers of a bird suffer from wear-and-tear and require to be molted. The timing of this molt - usually once a year but sometimes twice - varies with some species molting prior to moving to their winter grounds and others molting prior to returning to their breeding grounds.[68][69] Apart from physiological adaptations, migration sometimes requires behavioural changes such as flying in flocks to reduce the energy used in migration or the risk of predation.[70]
Evolutionary and ecological factors
Migration in birds is highly labile and is believed to have developed independently in many avian lineages.[71] While it is agreed that the behavioral and physiological adaptations necessary for migration are under genetic control, some authors have argued that no genetic change is necessary for migratory behavior to develop in a sedentary species because the genetic framework for migratory behavior exists in nearly all avian lineages.[72] This explains the rapid appearance of migratory behavior after the most recent glacial maximum.[73]
Theoretical analyses show that detours that increase flight distance by up to 20% will often be adaptive on aerodynamic grounds - a bird that loads itself with food to cross a long barrier flies less efficiently. However some species show circuitous migratory routes that reflect historical range expansions and are far from optimal in ecological terms. An example is the migration of continental populations of Swainson's thrush Catharus ustulatus, which fly far east across North America before turning south via Florida to reach northern South America; this route is believed to be the consequence of a range expansion that occurred about 10,000 years ago. Detours may also be caused by differential wind conditions, predation risk, or other factors.[74]
Climate change
Large scale climatic changes, as have been experienced in the past, are expected to have an effect on the timing of migration. Studies have shown a variety of effects including timing changes in migration, breeding[75] as well as population variations.[76][77]
Ecological effects
The migration of birds also aids the movement of other species, including those of ectoparasites such as ticks and lice,[78] which in turn may carry micro-organisms including those of concern to human health. Due to the global spread of avian influenza, bird migration has been studied as a possible mechanism of disease transmission, but it has been found not to present a special risk; import of pet and domestic birds is a greater threat.[79] Some viruses that are maintained in birds without lethal effects, such as the West Nile Virus may however be spread by migrating birds.[80] Birds may also have a role in the dispersal of propagules of plants and plankton.[81][82]
Some predators take advantage of the concentration of birds during migration. Greater noctule bats feed on nocturnal migrating passerines.[15] Some birds of prey specialize on migrating waders.[83]
Study techniques
Early studies on the timing of migration began in 1749 in Finland, with Johannes Leche of Turku collecting the dates of arrivals of spring migrants.[84]
Bird migration routes have been studied by a variety of techniques including the oldest, marking. Swans have been marked with a nick on the beak since about 1560 in England. Scientific ringing was pioneered by Hans Christian Cornelius Mortensen in 1899.[85] Other techniques include radar[86] and satellite tracking.[87]
Stable isotopes of hydrogen, oxygen, carbon, nitrogen, and sulphur can establish avian migratory connectivity between wintering sites and breeding grounds. Stable isotopic methods to establish migratory linkage rely on spatial isotopic differences in bird diet that are incorporated into inert tissues like feathers, or into growing tissues such as claws and muscle or blood.[88][89]
An approach to identify migration intensity makes use of upward pointing microphones to record the nocturnal contact calls of flocks flying overhead. These are then analyzed in a laboratory to measure time, frequency and species.[90]
An older technique to quantify migration involves observing the face of the moon towards full moon and counting the silhouettes of flocks of birds as they fly at night.[91][92]
Orientation behaviour studies have been traditionally carried out using variants of a setup known as the Emlen funnel, which consists of a circular cage with the top covered by glass or wire-screen so that either the sky is visible or the setup is placed in a planetarium or with other controls on environmental cues. The orientation behaviour of the bird inside the cage is studied quantitatively using the distribution of marks that the bird leaves on the walls of the cage.[93] Other approaches used in pigeon homing studies make use of the direction in which the bird vanishes on the horizon.[94]
Threats and conservation
Human activities have threatened many migratory bird species. The distances involved in bird migration mean that they often cross political boundaries of countries and conservation measures require international cooperation. Several international treaties have been signed to protect migratory species including the Migratory Bird Treaty Act of 1918 of the US.[95] and the African-Eurasian Migratory Waterbird Agreement[96]
The concentration of birds during migration can put species at risk. Some spectacular migrants have already gone extinct; during the passenger pigeon's (Ectopistes migratorius) migration the enormous flocks were a mile (1.6 km) wide, darkening the sky and 300 miles (480 km) long, taking several days to pass.[97]
Other significant areas include stop-over sites between the wintering and breeding territories.[98] A capture-recapture study of passerine migrants with high fidelity for breeding and wintering sites did not show similar strict association with stop-over sites.[99]
Hunting along migration routes threatens some bird species. The populations of Siberian cranes (Leucogeranus leucogeranus) that wintered in India declined due to hunting along the route, particularly in Afghanistan and Central Asia. Birds were last seen in their favourite wintering grounds in Keoladeo National Park in 2002.[100] Structures such as power lines, wind farms and offshore oil-rigs have also been known to affect migratory birds.[101] Other migration hazards include pollution, storms, wildfires, and habitat destruction along migration routes, denying migrants food at stopover points.[102] For example, in the East Asian–Australasian Flyway, up to 65% of key intertidal habitat at the Yellow Sea migration bottleneck has been destroyed since the 1950s.[103][104]
See also
- Ambelopoulia
- Winged Migration, 2001 documentary film
- Isoscapes
- Smithsonian Migratory Bird Center
References
- ↑ 1.0 1.1 1.2 Lincoln, F. C. (1979). Migration of Birds. Circular 16 (Fish and Wildlife Service).
- ↑ Cocker, Mark; Mabey, Richard (2005). Birds Britannica. Chatto & Windus. p. 315. ISBN 0-7011-6907-9.
- ↑ 3.0 3.1 White, 1898. pp. 27–28
- ↑ White, 1898. pp. 161–162
- ↑ Bewick, 1797. p. xvii
- ↑ Bewick, 1797. p. 300
- ↑ Bewick, 1797. pp. 302–303
- ↑ Peter Berthold, Hans-Günther Bauer, Valerie Westhead (2001). Bird Migration: A General Survey. Oxford: Oxford University Press. ISBN 0-19-850787-9.
- ↑ Sekercioglu, C.H. (2007). "Conservation ecology: area trumps mobility in fragment bird extinctions". Current Biology 17 (8): 283–286. doi:10.1016/j.cub.2007.04.045.
- ↑ Rolland, J., Jiguet, F., Jønsson, K.A., Condamine, F.L. & Morlon, H. (2014). "Settling down of seasonal migrants promotes bird diversification". Proceedings of the Royal Society B 281 (1784): 20140473. doi:10.1098/rspb.2014.0473.
- ↑ 11.0 11.1 11.2 11.3 11.4 Newton, I. (2008). The Migration Ecology of Birds. Elsevier. ISBN 978-0-12-517367-4.
- ↑ "Migration Basics". Hummingbirds.net. Retrieved 10 April 2014.
- ↑ Ramachandra, T.V. et al. (February 2011). "Environmental Impact Assessment of the National Large Solar Telescope Project and its Ecological Impact in Merak Area". p. 71. Retrieved 10 April 2014.
- ↑ Dondini, G., Vergari, S. (2000). "Carnivory in the greater noctule bat (Nyctalus lasiopterus) in Italy". Journal of Zoology 251 (2): 233–236. doi:10.1111/j.1469-7998.2000.tb00606.x.
- ↑ 15.0 15.1 Popa-Lisseanu, A. G., Delgado-Huertas, A., Forero, M. G., Rodriguez, A., Arlettaz, R. & Ibanez, C. (2007). Rands, Sean, ed. "Bats' Conquest of a Formidable Foraging Niche: The Myriads of Nocturnally Migrating Songbirds". PLoS ONE 2 (2): e205. doi:10.1371/journal.pone.0000205. PMC 1784064. PMID 17299585.
- ↑ Ibáñez, C., Juste, J., García-Mudarra, J. L., Agirre-Mendi, P. T. (2001). "Bat predation on nocturnally migrating birds". PNAS 98 (17): 9700–9702. doi:10.1073/pnas.171140598. PMC 55515. PMID 11493689.
- ↑ Chan K (2001). "Partial migration in Australian landbirds: a review". Emu 101 (4): 281–292. doi:10.1071/MU00034.
- ↑ Boland, J. M. (1990). "Leapfrog migration in North American shorebirds: intra- and interspecific examples" (PDF). The Condor 92 (2): 284–290. doi:10.2307/1368226. JSTOR 1368226.
- ↑ Berthold, Peter (2001). Bird Migration: A General Survey. Oxford University Press. p. 67.
- ↑ Panov, Ilya N. (2011). "Overlap between moult and autumn migration in passerines in northern taiga zone of Eastern Fennoscandia" (PDF). Avian Ecology and Behaviour 19: 33–64.
- ↑ Hummel D., Beukenberg M. (1989). "Aerodynamische Interferenzeffekte beim Formationsfl ug von Vogeln". J. Ornithol 130: 15–24. doi:10.1007/BF01647158.
- ↑ Cutts, C. J. & J R Speakman (1994). "Energy savings in formation flight of Pink-footed Geese" (PDF). J. Exp. Biol. 189 (1): 251–261. PMID 9317742.
- ↑ Geroudet, P. (1954). "Des oiseaux migrateurs trouvés sur la glacier de Khumbu dans l'Himalaya". Nos Oiseaux 22: 254.
- ↑ Swan, L. W. (1970). "Goose of the Himalayas". Nat. Hist. 79 (10): 68–75.
- ↑ Dorst, J. (1963). The migration of birds. Houghton Mifflin Co., Boston. p. 476.
- ↑ Eastwood, E. & G. C. Rider. (1965). "Some radar measurements of the altitude of bird flight". British Birds 58: 393–426.
- ↑ Williams, G. G. (1950). "Weather and spring migration". Auk 67: 52–65. doi:10.2307/4080769.
- ↑ Dark-Eyed Junco
- ↑ American Goldfinch
- ↑ Green, Martin (1999). "The Riddle of the White Sea". Geese.org. Retrieved 10 April 2014.
- ↑ "Species factsheet: Dunlin Calidris alpina". BirdLife International. 2014. Retrieved 19 June 2014.
- ↑ "Species factsheet: Western Sandpiper Calidris mauri". BirdLife International. 2014. Retrieved 19 June 2014.
- ↑ "Species factsheet: Semipalmated Sandpiper Calidris pusilla". BirdLife International. 2014. Retrieved 19 June 2014.
- ↑ Sprague, A. J., D. J. Hamilton, and A. W. Diamond (2008). "Site Safety and Food Affect Movements of Semipalmated Sandpipers (Calidris pusilla) Migrating Through the Upper Bay of Fundy" (PDF). Avian Conservation and Ecology 3 (2).
- ↑ Kathleen E. Clark, Lawrence J. Niles and Joanna Burger. "Abundance and Distribution of Migrant Shorebirds in Delaware Bay" (PDF). The Condor (95): 694–705.
- ↑ Gill, Robert E. Jr., Theunis Piersma, Gary Hufford, Rene Servranckx, Adrian Riegen (2005). "Crossing the ultimate ecological barrier: evidence for an 11,000 km-long nonstop flight from Alaska to New Zealand and Eastern Australia by Bar-tailed Godwits". The Condor 107 (1): 1–20. doi:10.1650/7613.
- ↑ Cramp, S., ed. (1985). Birds of the Western Palearctic. pp. 87–100. ISBN 0-19-857507-6.
- ↑ Pyle, Peter (2001). "Seabirds" (PDF). Circular 1198. USGS. p. 154. Retrieved 19 June 2014.
- ↑ Anon (18 April 2002). "Oldest bird clocks 5 million miles". CNN.com. Retrieved 31 March 2013.
- ↑ Maanen, E. van; Goradze, I.; Gavashelishvili, A.; Goradze, R. (2001). "Opinion: Trapping and hunting of migratory raptors in western Georgia". Bird Conservation International 11 (2): 77–92. doi:10.1017/S095927090100017X.
- ↑ Gensbol, B; (1984) Collins Guide to the Birds of Prey of Britain and Europe, p.28
- ↑ Schmaljohann, Heiko, Felix Liechti and Bruno Bruderer (2007). "Songbird migration across the Sahara: the non-stop hypothesis rejected!". Proceedings of the Royal Society B 274 (1610): 735–739. doi:10.1098/rspb.2006.0011. PMC 2197203. PMID 17254999.
- ↑ Rattenborg, N.C., Mandt, B.H., Obermeyer, W.H., Winsauer, P.J., Huber, R. (2004). "Migratory Sleeplessness in the White-Crowned Sparrow (Zonotrichia leucophrys gambelii)". PLoS Biol. 2 (7): e212. doi:10.1371/journal.pbio.0020212. PMC 449897. PMID 15252455.
- ↑ "British Wildlife Recordings: Chaffinch". British Library. Retrieved 10 April 2014.
- ↑ Cocker, 2005. p. 378
- ↑ 46.0 46.1 Cocker, 2005. p. 326
- ↑ Boyle, W. A.; Conway, C. J.; Bronstein, J. L. (2011). "Why do some, but not all, tropical birds migrate? A comparative study of diet breadth and fruit preference" (PDF). Evolutionary Ecology 25: 219–236. doi:10.1007/s10682-010-9403-4.
- ↑ Kreft, Stefan (23 June 2004). "The Fourth Dimension: An Overview of Altitudinal Migration" (PDF). 25th Annual Bonn Convention, Berlin. Retrieved 27 March 2013.
- ↑ Cocker, 2005. p. 455
- ↑ Somveille M, Manica A, Butchart SHM, Rodrigues ASL (2013). "Mapping Global Diversity Patterns for Migratory Birds". PLOS one 8 (8): e70907. doi:10.1371/journal.pone.0070907.
- ↑ Newton, Ian (2010). "13. Large-Scale Movement Patterns". The Migration Ecology of Birds. Academic Press. pp. 396, and throughout.
- ↑ Helm B, Gwinner E (2006). "Migratory Restlessness in an Equatorial Nonmigratory Bird". PLoS Biol. 4 (4): e110. doi:10.1371/journal.pbio.0040110. PMC 1420642. PMID 16555925.
- ↑ Fusani, L.; Cardinale, L.; Carere, C.; Goymann, W. (2009). "Stopover decision during migration: physiological conditions predict nocturnal restlessness in wild passerines". Biology Letters. doi:10.1098/rsbl.2008.0755.
- ↑ Nievergelt, F.; Liechti, F.; Bruderer, B. (1999). "MIGRATORY DIRECTIONS OF FREE-FLYING BIRDS VERSUS ORIENTATION IN REGISTRATION CAGES" (PDF). Journal of Experimental Biology 202 (16): 2225–2231.
- ↑ Diego Rubolini, Fernando Spina and Nicola Saino (2004). "Protandry and sexual dimorphism in trans-Saharan migratory birds". Behavioral Ecology 15 (4): 592–601. doi:10.1093/beheco/arh048.
- ↑ Edwards, Darryl B.; Forbes, Mark R. (2007). "Absence of protandry in the spring migration of a population of Song Sparrows Melospiza melodia". Ibis 149 (4): 715–720. doi:10.1111/j.1474-919X.2007.00692.x.
- ↑ Walraff, H. G. (2005). Avian Navigation: Pigeon Homing as a Paradigm. Springer.
- ↑ Ketterson, E.D. and V. Nolan Jr. (1990). "Site attachment and site fidelity in migratory birds: experimental evidence from the field and analogies from neurobiology.". In E. Gwinner. Bird Migration. Springer Verlag. pp. 117–129. Archived from the original (PDF) on 2010-02-10.
- ↑ Thorup, Kasper, Thomas Alerstam, Mikael Hake and Nils Kjelle (2003). "Bird orientation: compensation for wind drift in migrating raptors is age dependent". Proceedings of the Royal Society B 270 (Suppl 1): S8–S11. doi:10.1098/rsbl.2003.0014. PMC 1698035. PMID 12952622.
- ↑ Wiltschko, W., U. Munro, H. Ford & R. Wiltschko (2006). "Bird navigation: what type of information does the magnetite-based receptor provide?". Proceedings of the Royal Society B 273 (1603): 2815–20. doi:10.1098/rspb.2006.3651. PMC 1664630. PMID 17015316.
- ↑ Heyers D, Manns M, Luksch H, Güntürkün O, Mouritsen H (2007). Iwaniuk, Andrew, ed. "A Visual Pathway Links Brain Structures Active during Magnetic Compass Orientation in Migratory Birds". PLoS ONE 2 (9): e937. doi:10.1371/journal.pone.0000937. PMC 1976598. PMID 17895978.
- ↑ Deutschlander, ME, Phillips, JB, Borland, SC (1999). "The case for light-dependent magnetic orientation in animals" (PDF). J.Exp. Biol. 202 (8): 891–908. PMID 10085262.
- ↑ Thorup, Kasper (2004). "Reverse migration as a cause of vagrancy" (PDF). Bird Study 51: 228–238. doi:10.1080/00063650409461358.
- ↑ Kasper Thorup, Thomas Alerstam, Mikael Hake, Nils Kjellén (2003). "Bird orientation: compensation for wind drift in migrating raptors is age dependent". Proc. Royal Soc. London B 270 (Suppl 1): S8–S11. doi:10.1098/rsbl.2003.0014. PMC 1698035. PMID 12952622.
- ↑ Guillemain, M., Sadoul, N. and Simon, G. (2005), European flyway permeability and abmigration in Teal Anas crecca, an analysis based on ringing recoveries. Ibis, 147: 688–696. doi:10.1111/j.1474-919X.2005.00446.x
- ↑ "Operation migration".
- ↑ "Wisconsin Whooping Crane Management Plan" (PDF). Wisconsin Department of Natural Resources. 6 December 2006.
- ↑ Rohwer S; Butler LK & DR Froehlich (2005). "Ecology and Demography of East-West Differences in Molt Scheduling of Neotropical Migrant Passerines". In Greenberg R & Marra PP. Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press. p. 94. ISBN 0-8018-8107-2.
- ↑ Hedenström, A. (2008). "Adaptations to migration in birds: behavioural strategies, morphology and scaling effects". Philosophical Transactions of the Royal Society B 363 (1490): 287–299. doi:10.1098/rstb.2007.2140. PMC 2606751. PMID 17638691.
- ↑ Weber, Jean-Michel (2009). "The physiology of long-distance migration: extending the limits of endurance metabolism". J. Exp. Biol. 212 (Pt 5): 593–597. doi:10.1242/jeb.015024. PMID 19218508.
- ↑ Pulido, F. (2007). "The genetics and evolution of avian migration". BioScience 57: 165–174. doi:10.1641/b570211.
- ↑ J. Rappole, B. Helm, M. Ramos (2003). "An integrative framework for understanding the origin and evolution of avian migration". Journal of Avian Biology 34: 125. doi:10.1034/j.1600-048x.2003.03170.x.
- ↑ B. Mila, T. Smith, R. Wayne. (2006). "Postglacial population expansion drives the evolution of long-distance avian migration in a songbird". Evolution 60: 2403–2409. doi:10.1554/06-153.1.
- ↑ Alerstam, Thomas (2001). "Detours in bird migration" (PDF). Journal of Theoretical Biology 209: 319–331. doi:10.1006/jtbi.2001.2266.
- ↑ Jenni L. & Kery M. (2003). "Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants". Proceedings of the Royal Society B 270: 1467. doi:10.1098/rspb.2003.2394.
- ↑ Both, Christiaan; Sandra Bouwhuis; C. M. Lessells; Marcel E. Visser (2006-05-04). "Climate change and population declines in a long-distance migratory bird". Nature 441 (7089): 81–83. doi:10.1038/nature04539. ISSN 0028-0836. PMID 16672969.
- ↑ Wormworth, J.; Mallon, K. (2006). Bird Species and Climate Change: The Global Status Report version 1.0. WWF.
- ↑ Smith RP Jr, Rand PW, Lacombe EH, Morris SR, Holmes DW, Caporale DA (1996). "Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease". J. Infect. Dis. 174 (1): 221–4. doi:10.1093/infdis/174.1.221. PMID 8656000.
- ↑ Rappole, J.H., Hubálek, Zdenek (2006). "Birds and Influenza H5N1 Virus Movement to and within North America". Emerging Infectious Diseases 12: 10. doi:10.3201/eid1210.051577. hdl:10088/875.
- ↑ Rappole, J.H., Derrickson, S.R., Hubalek, Z. (2000). "Migratory birds and spread of West Nile virus in the Western Hemisphere". Emerging Infectious Diseases 6 (4): 319–328. doi:10.3201/eid0604.000401. PMC 2640881. PMID 10905964. hdl:10088/364.
- ↑ Figuerola, O.; Green, A.J. (2002). "Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies". Freshwater Biology 47 (3): 483–494. doi:10.1046/j.1365-2427.2002.00829.x.
- ↑ Cruden, R. W. (1966). "Birds as Agents of Long-Distance Dispersal for Disjunct Plant Groups of the Temperate Western Hemisphere". Evolution (Evolution, Vol. 20, No. 4) 20 (4): 517–532. doi:10.2307/2406587. JSTOR 2406587.
- ↑ Ydenberg, Ronald C.; Butler, Robert W.; Lank, David B. ; Smith, Barry D.; Ireland, J. (2004). "Western sandpipers have altered migration tactics as peregrine falcon populations have recovered" (PDF). Proceedings of the Royal Society B 271 (1545): 1263–1269 1263. doi:10.1098/rspb.2004.2713. PMC 1691718. PMID 15306350.
- ↑ Greenwood, Jeremy J. D. (2007). "Citizens, science and bird conservation". J. Ornithol . 148 (Suppl 1): S77–S124. doi:10.1007/s10336-007-0239-9.
- ↑ Spencer, R. (1985) Marking. In: Campbell. B. & Lack, E. 1985. A dictionary of birds. British Ornithologists' Union. London, pp. 338–341.
- ↑ "Radar Ornithology: Introduction". Clemson University Radar Ornithology Laboratory. Retrieved 15 June 2014.
- ↑ "Tracking Cuckoos to Africa ... and back again". British Trust for Ornithology. Retrieved 15 June 2014.
- ↑ Keith Hobson, Leonard Wassenaar (1997). "Linking breeding and wintering grounds of neotropical migrant songbirds using stable hydrogen isotopic analysis of feathers". Oecologia 109: 142–148. doi:10.1007/s004420050068.
- ↑ Gabriel Bowen, Leonard Wassenaar, Keith Hobson (2005). "Global application of stable hydrogen and oxygen isotopes to wildlife forensics". Oecologia 143 (3): 337–348. doi:10.1007/s00442-004-1813-y. PMID 15726429.
- ↑ Farnsworth, A., Gauthreaux, S.A., and van Blaricom, D. (2004). "A comparison of nocturnal call counts of migrating birds and reflectivity measurements on Doppler radar" (PDF). Journal of Avian Biology 35 (4): 365–369. doi:10.1111/j.0908-8857.2004.03180.x.
- ↑ Liechti, F. (1996). Instructions to count nocturnal bird migration by watching the full moon. Schweizerische Vogelwarte, CH-6204 Sempach, Switzerland.
- ↑ Lowery, G.H. (1951). "A quantitative study of the nocturnal migration of birds". University Kan. Pub. Mus. Nat. Hist. 3: 361–472.
- ↑ Emlen, S. T. and Emlen, J. T. (1966). "A technique for recording migratory orientation of captive birds". Auk 83: 361–367. doi:10.2307/4083048.
- ↑ Alerstam, 1993. p.352
- ↑ "Migratory bird Treaty 16 USC 703-711; 40 Stat. 755". Legal Information Institute (LII). Cornell Law School.
- ↑ "African-Eurasian Migratory Waterbird Agreement".
- ↑ "The Passenger Pigeon". Smithsonian Institution. Retrieved 2013-05-24.
- ↑ Shimazaki, Hiroto; Masayuki Tamura and Hiroyoshi Higuchi (2004). "Migration routes and important stopover sites of endangered oriental white storks (Ciconia boyciana) as revealed by satellite tracking" (PDF). Mem Natl Inst. Polar Res., Spec. Issue 58: 162–178.
- ↑ Catry, P., Encarnacao, V., Araujo, A., Fearon, P., Fearon, A., Armelin, M. & Delaloye, P. (2004). "Are long-distance migrant passerines faithful to their stopover sites?" (PDF). Journal of Avian Biology 35 (2): 170–181. doi:10.1111/j.0908-8857.2004.03112.x.
- ↑ "Siberian Crane fact sheet".
- ↑ "Fish and Wildlife Service- Bird Mortality Fact sheet" (PDF).
- ↑ Mayntz, Melissa. "Threats to Migrating Birds". About.com Birding. Retrieved 19 June 2014.
- ↑ Murray N. J., Clemens R. S., Phinn S. R., Possingham H. P. & Fuller R. A. (2014) Tracking the rapid loss of tidal wetlands in the Yellow Sea. Frontiers in Ecology and the Environment 12, 267-72. doi: 10.1890/130260
- ↑ MacKinnon, J.; Verkuil, Y.I.; Murray, N.J. (2012), IUCN situation analysis on East and Southeast Asian intertidal habitats, with particular reference to the Yellow Sea (including the Bohai Sea), Occasional Paper of the IUCN Species Survival Commission No. 47, Gland, Switzerland and Cambridge, UK: IUCN, p. 70, ISBN 978-2-8317-1255-0
Further reading
- Alerstam, Thomas (2001). "Detours in bird migration" (PDF). Journal of Theoretical Biology 209: 319–331. doi:10.1006/jtbi.2001.2266.
- Alerstam, Thomas (1993). Bird Migration. Cambridge University Press. ISBN 0-521-44822-0. (first published 1982 as Fågelflyttning, Bokförlaget Signum)
- Berthold, Peter (2001). Bird Migration: A General Survey (2nd ed.). Oxford University Press. ISBN 0-19-850787-9.
- Bewick, Thomas (1797–1804). History of British Birds (1847 ed.). Newcastle: Beilby and Bewick.
- Dingle, Hugh (1996). Migration: The Biology of Life on The Move. Oxford University Press.
- Hobson, Keith; Wassenaar, Leonard (2008). Tracking Animal Migration with Stable Isotopes. Academic Press. ISBN 978-0-12-373867-7.
- Weidensaul, Scott (1999). Living On the Wind: Across the Hemisphere With Migratory Birds. Douglas & McIntyre.
- White, Gilbert (1898) [1789]. The Natural History of Selborne. Walter Scott.
External links
- Dedicated issue of Philosophical Transactions B on Adaptation to the Annual Cycle.
- Route of East Asian Migratory Flyaway Olango Wildlife Sanctuary as a refuelling station of migratory birds
- Migraction.net - Interactive database with real-time information on bird migration
- Migration Ecology Group, Lund University, Sweden
- Migrate.ou.edu - Migration Interest Group: Research Applied Toward Education, USA
- Trektellen.nl - Migration counts and ringing records: The Netherlands, Belgium, Great Britain, France, Germany, Spain and Portugal
- Canadian Migration Monitoring Network (Co-ordinates bird migration monitoring stations across Canada)
- Bird Research by Science Daily- includes several articles on bird migration
- The Nature Conservancy's Migratory Bird Program
- The Compasses of Birds - a review from the Science Creative Quarterly
- BBC Supergoose - satellite tagging of light-bellied brent geese
- Soaring with Fidel - follow the annual migration of ospreys from Cape Cod to Cuba to Venezuela
- Birder's Journal: A Morning With Migrants Nationalgeographic.com
- Bat predation on migrating birds
- Global Register of Migratory Species - features not only birds, but other migratory vertebrates such as fishes
- eBird.com Occurrence Maps - Occurrence maps of migrations of various species in United States
- Smithsonian Migratory Bird Center - "Fostering greater understanding, appreciation, and protection of the grand phenomenon of bird migration."
| ||||||||||||||||||||||||||||||||||||||||||||||||||||