Bicornin
Bicornin
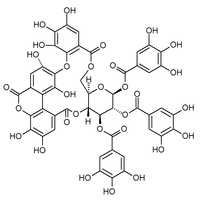 |
| Identifiers |
| |
124854-12-2 |
| ChemSpider |
28190824 |
InChI=1S/C48H32O30/c49-17-1-11(2-18(50)29(17)58)42(65)76-40-38-26(72-48(78-44(67)13-5-21(53)31(60)22(54)6-13)41(40)77-43(66)12-3-19(51)30(59)20(52)4-12)10-71-45(68)16-9-23(55)32(61)35(64)36(16)73-37-25(57)8-14-27(34(37)63)28-15(47(70)74-38)7-24(56)33(62)39(28)75-46(14)69/h1-9,26,38,40-41,48-64H,10H2/t26-,38-,40-,41-,48+/m1/s1
Key: DOTJYWQAPHIAIF-TWNAEOARSA-N InChI=1/C48H32O30/c49-17-1-11(2-18(50)29(17)58)42(65)76-40-38-26(72-48(78-44(67)13-5-21(53)31(60)22(54)6-13)41(40)77-43(66)12-3-19(51)30(59)20(52)4-12)10-71-45(68)16-9-23(55)32(61)35(64)36(16)73-37-25(57)8-14-27(34(37)63)28-15(47(70)74-38)7-24(56)33(62)39(28)75-46(14)69/h1-9,26,38,40-41,48-64H,10H2/t26-,38-,40-,41-,48+/m1/s1
Key: DOTJYWQAPHIAIF-TWNAEOARBX
|
| Jmol-3D images |
Image |
c1c(cc(c(c1O)O)O)C(=O)O[C@@H]2[C@H]3[C@@H](COC(=O)c4cc(c(c(c4Oc5c(cc6c(c5O)c7c(cc(c(c7oc6=O)O)O)C(=O)O3)O)O)O)O)O[C@H]([C@@H]2OC(=O)c8cc(c(c(c8)O)O)O)OC(=O)c9cc(c(c(c9)O)O)O
|
| Properties |
| |
C48H32O30 |
| Molar mass |
1088.71 g/mol |
Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) |
|
| Infobox references |
|
|
Bicornin is an ellagitannin found in the Myrtales Trapa bicornis (water caltrop)[1] and Syzygium aromaticum (clove).[2]
The molecule contains a luteic acid group.[3]
References
- ↑ Bicornin, a new hydrolyzable tannin from Trapa bicornis, and revised structure of alnusiin. Yoshida T, Yazaki K, Memon M.U, Maruyama I, Kurokawa K and Okuda T, Heterocycles, 1989, volume 29, number 5, pages 861-864
INIST:6780591
- ↑ Hydrolysable Tannins Isolated from Syzygium aromaticum: Structure of a New C-Glucosidic Ellagitannin and Spectral Features of Tannins with a Tergalloyl Group. Li-Ming Bao, Eerdunbayaer, Akiko Nozaki, Eizo Takahashi, Keinosuke Okamoto, Hideyuki Ito and Tsutomu Hatano, Heterocycles, 2012, Volume 85, Number 2, pages 365-381, doi:10.3987/COM-11-12392
- ↑ Structures of alnusiin and bicornin, new hydrolyzable tannins having a monolactonized tergalloyl group. Yoshida T, Yazaki K, Memon M.U, Maruyama I, Kurokawa K, Shingu T and Okuda T, Chemical and pharmaceutical bulletin, 1989, volume 37, number 10, pages 2655-2660,
INIST:19467830
(abstract)
External links
|
|---|
| | Moieties | |
|---|
| | Lactones | |
|---|
| | Monomers |
- Acetonyl geraniin
- Alnusiin
- Bicornin
- Carlesiin
- Casuarictin
- Emblicanin A and B
- Euscaphinin
- Galloyl pedunculagin
- Grandinin
- Helioscopinin B
- Jolkinin
- Lagerstannin A, B and C
- Macranganin
- Myrobalanitannin
- Nupharin A, B, C, D, E and F
- Pedunculagin
- Punicalagin
- Punigluconin
- Phyllanemblinin A, B, C, D, E and F
- Punicalin
- Roburin E
- Rugosin E
- Sanguiin H-5
- Stenophyllanin A, B and C
- Strictinin
- Tellimagrandin I and II
- Teracatain
- Terchebulin
- Terflavin A and B
- Tergallic acid
- Tergallic acid dilactone
C-glycosidic ellagitannins | |
|---|
| | |
|---|
| Transformed ellagitannins | | |
|---|
| molecules with Elaeocarpusinic acid |
- Elaeocarpusin
- Helioscopin B
- Mallojaponin (1-O-Galloyl-2,4-elaeocarpusinoyl-3,6-(R)-valoneayl-beta-D-glucose)
|
|---|
|
|---|
|
|---|
| | Oligomers | |
|---|
| | Other | |
|---|
|
