Beta-ketoacyl-acyl-carrier-protein synthase I
| 3-oxoacyl-[acyl-carrier-protein] synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.3.1.41 | ||||||||
| CAS number | 9077-10-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a beta-ketoacyl-acyl-carrier-protein synthase I (EC 2.3.1.41) is an enzyme that catalyzes the chemical reaction
- an acyl-[acyl-carrier-protein] + malonyl-[acyl-carrier-protein]
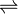 a 3-oxoacyl-[acyl-carrier-protein] + CO2 + [acyl-carrier-protein]
a 3-oxoacyl-[acyl-carrier-protein] + CO2 + [acyl-carrier-protein]
Thus, the two substrates of this enzyme are [[acyl-[acyl-carrier-protein]]] and [[malonyl-[acyl-carrier-protein]]], whereas its 3 products are [[3-oxoacyl-[acyl-carrier-protein]]], CO2, and acyl carrier protein.
This enzyme belongs to the family of transferases, specifically those acyltransferases transferring groups other than aminoacyl groups. The systematic name of this enzyme class is acyl-[acyl-carrier-protein]:malonyl-[acyl-carrier-protein] C-acyltransferase (decarboxylating). Other names in common use include beta-ketoacyl-ACP synthase I, beta-ketoacyl synthetase, beta-ketoacyl-ACP synthetase, beta-ketoacyl-acyl carrier protein synthetase, beta-ketoacyl-[acyl carrier protein] synthase, beta-ketoacylsynthase, condensing enzyme, 3-ketoacyl-acyl carrier protein synthase, fatty acid condensing enzyme, acyl-malonyl(acyl-carrier-protein)-condensing enzyme, acyl-malonyl acyl carrier protein-condensing enzyme, beta-ketoacyl acyl carrier protein synthase, 3-oxoacyl-[acyl-carrier-protein] synthase, 3-oxoacyl:ACP synthase I, KASI, KAS I, FabF1, and FabB. This enzyme participates in fatty acid biosynthesis.
Structural studies
As of late 2007, 56 structures have been solved for this class of enzymes, with PDB accession codes 1B3N, 1DD8, 1E5M, 1EBL, 1EK4, 1F91, 1FJ4, 1FJ8, 1G5X, 1H4F, 1HN9, 1HND, 1HNH, 1HNJ, 1HNK, 1HZP, 1J3N, 1KAS, 1M1M, 1MZJ, 1MZS, 1OX0, 1OXH, 1TQY, 1U6E, 1U6S, 1UB7, 1W0I, 1ZOW, 2AHB, 2AJ9, 2ALM, 2AQ7, 2AQB, 2BUH, 2BUI, 2BYW, 2BYX, 2BYY, 2BYZ, 2BZ3, 2BZ4, 2C9H, 2EBD, 2EFT, 2GFV, 2GFW, 2GFX, 2GFY, 2GP6, 2GQD, 2GYO, 2IWY, 2IWZ, 2IX4, and 2PFF.
References
- Alberts AW, Majerus PW and Vagelos PR (1969). "Acetyl-CoA acyl carrier protein transacylase". Methods Enzymol. Methods in Enzymology 14: 50–53. doi:10.1016/S0076-6879(69)14009-4. ISBN 978-0-12-181871-5.
|chapter=ignored (help) - Prescott DJ, Vagelos PR (1972). "Acyl carrier protein". Adv. Enzymol. Relat. Areas. Mol. Biol. 36: 269–311. PMID 4561013.
- Toomey RE, Wakil SJ (1966). "Studies on the mechanism of fatty acid synthesis. XVI. Preparation and general properties of acyl-malonyl acyl carrier protein-condensing enzyme from Escherichia coli". J. Biol. Chem. 241 (5): 1159–65. PMID 5327099.
- D'Agnolo G, Rosenfeld IS, Vagelos PR (1975). "Multiple forms of beta-ketoacyl-acyl carrier protein synthetase in Escherichia coli". J. Biol. Chem. 250 (14): 5289–94. PMID 237914.
- Garwin JL, Klages AL, Cronan JE Jr (1980). "Structural, enzymatic, and genetic studies of beta-ketoacyl-acyl carrier protein synthases I and II of Escherichia coli". J. Biol. Chem. 255 (24): 11949–56. PMID 7002930.
- Wang H, Cronan JE (2004). "Functional replacement of the FabA and FabB proteins of Escherichia coli fatty acid synthesis by Enterococcus faecalis FabZ and FabF homologues". J. Biol. Chem. 279 (33): 34489–95. doi:10.1074/jbc.M403874200. PMID 15194690.
- Neidhardt, F.C. (Ed.), Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed., vol. 1, ASM Press, Washington, DC, 1996, p. 612-636.